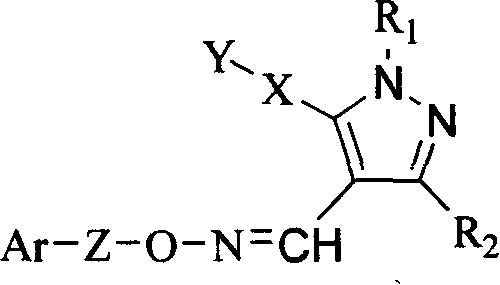
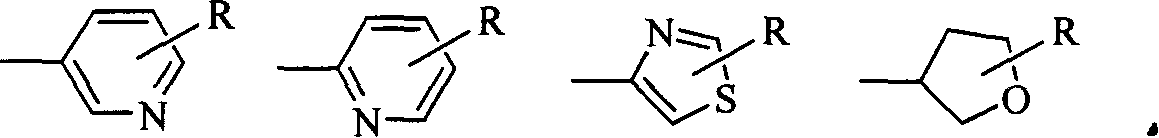
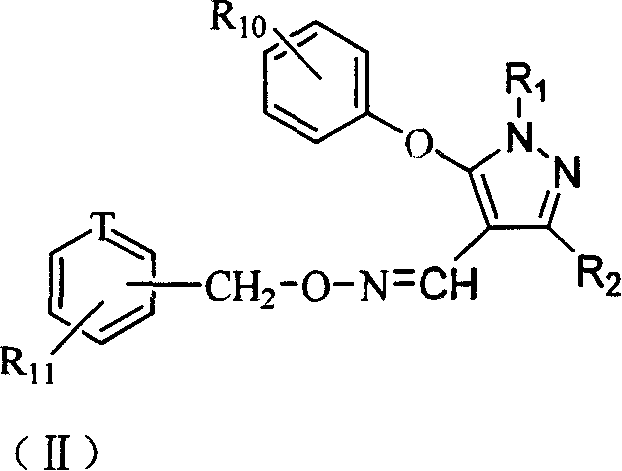
Pyrazol oxime ether derivatives and preparation process and use thereof
A technology for pyrazole oxime ethers and derivatives, applied in the field of pyrazole oxime ether derivatives and their preparation and application, to achieve high insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of compound 1:
[0039]
[0040] (1) Synthesis of 1,3-dimethyl-5-hydroxypyrazole
[0041]
[0042] 4.6g (0.1mol) methylhydrazine is added dropwise in the ethanolic solution of 11.6g (0.1mol) methyl acetoacetate, the process of dropping is cooled with ice-water bath, because the reaction process exothermic, the rate of addition depends on the reaction temperature at that time, It is advisable to control the temperature not to exceed 40°C, and then stir and react at room temperature for 6 hours. After the reaction, the solvent was removed below 60°C to obtain 8.4 g of light orange solid powder with a yield of 75%.
[0043] (2) Synthesis of 1,3-dimethyl-4-formaldehyde-5-chloropyrazole
[0044]
[0045] Below 10°C, 32ml (0.35mol) POCl 3 Add dropwise into DMF under stirring, after the dropwise addition is completed and stir for ten minutes, divide 5.6g (0.05mol) of 1,3-dimethyl-5-hydroxypyrazole into the above mixture gradually, and the above reaction m...
Embodiment 2
[0054] Synthesis of compound 17
[0055]
[0056] (1) Synthesis of methyl 6-bromomethylnicotinate
[0057]
[0058] Add 1.52g (0.01mol) methyl 6-methylnicotinate, 2.67g (0.015mol) NBS and 0.1g benzoyl peroxide to a four-neck flask equipped with mechanical stirring and reflux condenser, heat to reflux, After 16 hours of reaction, the reaction ended. Suction filtration, the filtrate was washed with saturated sodium carbonate solution, and the organic phase was washed with anhydrous MgSO 4 Drying, precipitation at 60°C, and column chromatography yielded 0.91 g of the product as a light pink solid powder with a yield of 39.7%.
[0059] 1 H NMR (CDCl 3 )δ (ppm) 300MHz: 3.97 (s, 3H, -COOCH 3 ), 4.59 (s, 2H, BrCH 2 -), 7.53-7.55 (d, 1H, Py-H), 8.29-8.32 (d, 1H, Py-H), 9.17 (d, 1H, Py-H).
[0060] (2) Synthesis of target compound 17
[0061]
[0062] Add 0.49g (2mmol) 1.3-dimethyl-5-(4-methyl)phenoxy-4-pyrazolecarbaldehyde oxime to a four-necked flask equipped with a ...
Embodiment 3
[0069] Synthesis of compound 31
[0070]
[0071] (1) Synthesis of tert-butyl 6-methylnicotinate
[0072]
[0073] Dissolve 1.51g (0.01mol) of methyl 6-methylnicotinate in 10ml of anhydrous diethyl ether, stir at room temperature under electromagnetic stirring, N 2 Next, add the above solution dropwise to a mixture of 40ml of anhydrous ether and 1.68g (0.015mol) of potassium tert-butoxide, and finish the dropwise addition in about 30 minutes, then continue to react at room temperature for about 30 minutes, and the reaction ends. The reaction solution passes through the neutral Al 2 o 3 Suction filtration and desolvation obtained 0.90 g of a colorless liquid product with a yield of 46.6%.
[0074] 1 H NMR (CDCl 3 )δ(ppm)300MHz: 1.60(s, 9H, -COOC(CH 3 ) 3 ), 2.61 (s, 3H, Py-CH 3 ), 7.19-7.22 (d, 1H, Py-H), 8.10-8.13 (d, 1H, Py-H), 9.17 (d, 1H, Py-H).
[0075] (2) Synthesis of tert-butyl 6-bromomethylnicotinate
[0076]
[0077] Add 1.93g (0.01mol) tert-butyl 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com