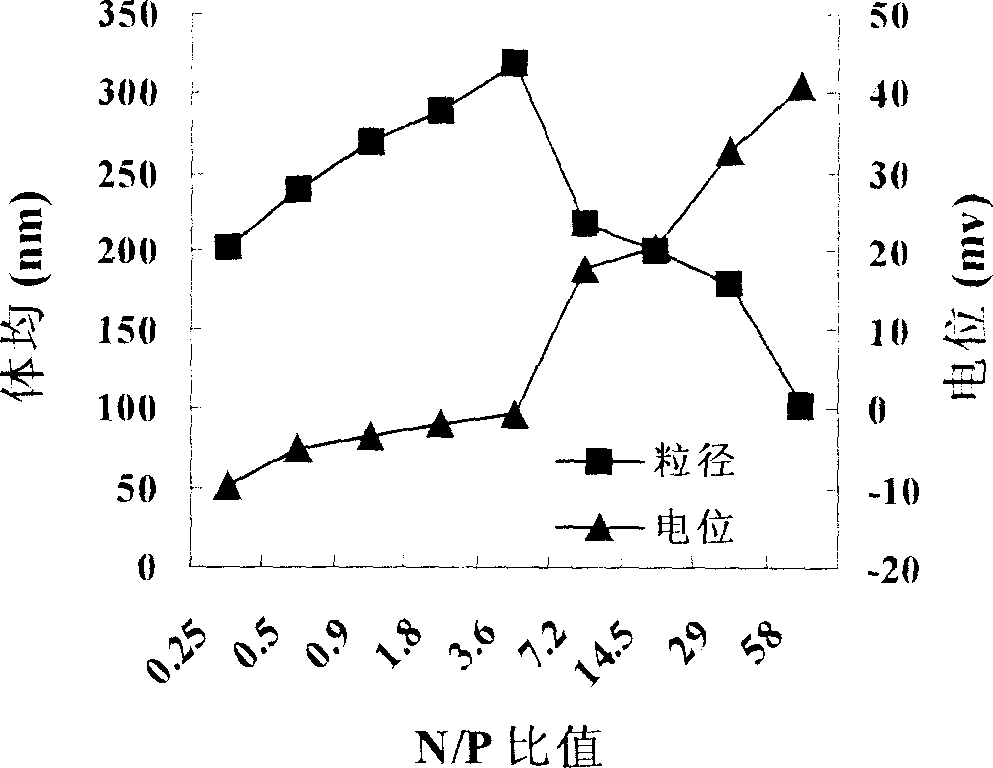
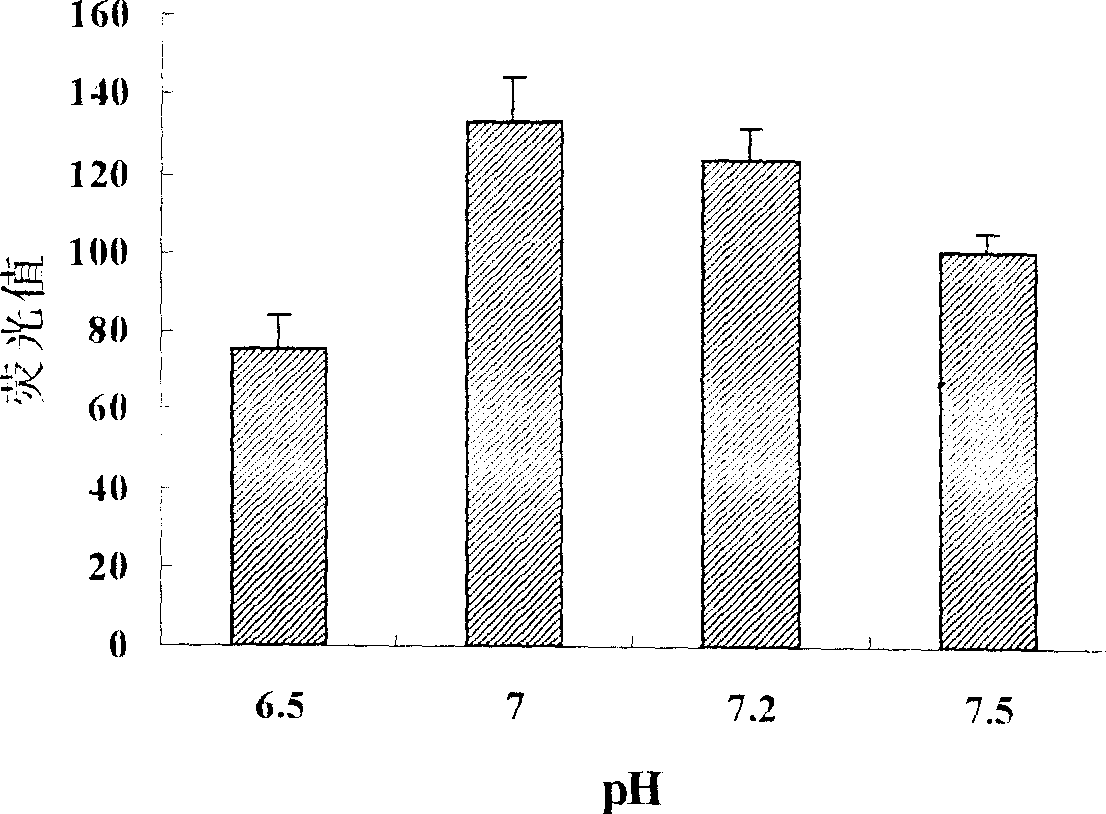
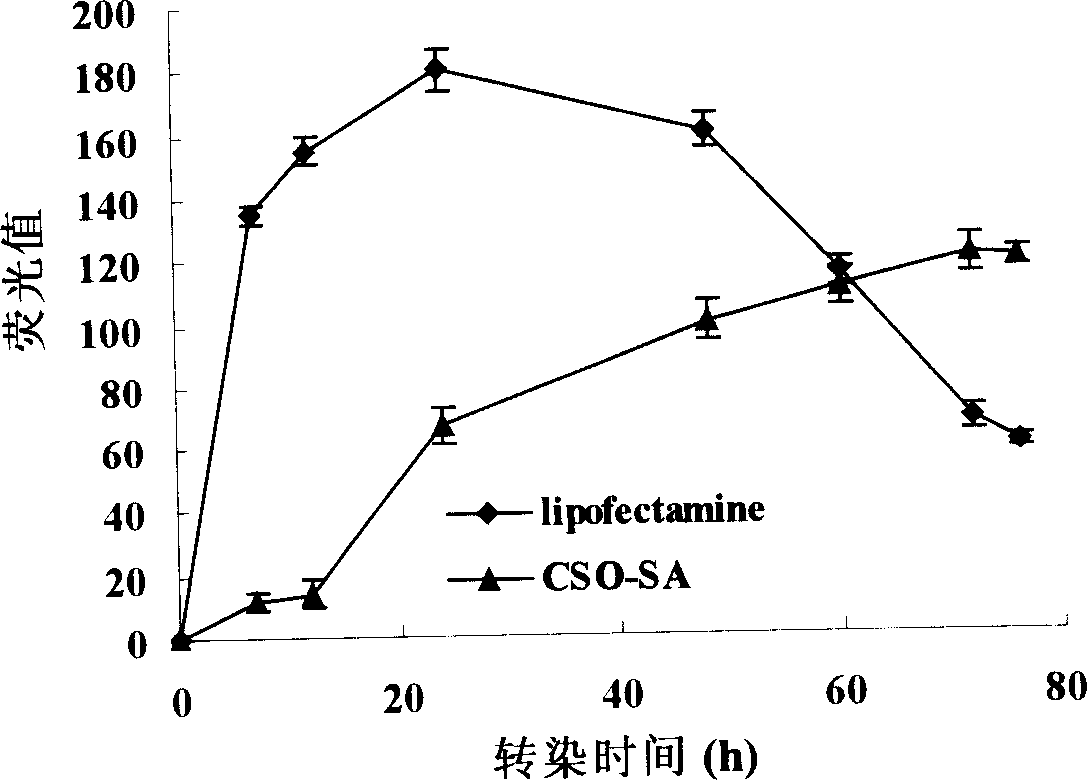
Non-viral gene transfection carrier and its preparation method and use
A gene transfection carrier and gene transfection technology, applied in the field of gene transfection ability, can solve the problems of cytotoxicity, low transfection efficiency, and low transfection efficiency, and achieve low toxicity, improved hydrophobicity, and transfection efficiency exact effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: a kind of synthetic method of chitosan-fatty acid graft:
[0032] Take 0.4 g of chitosan oligosaccharides with average molecular weights of 1.5, 10, 19, and 51 kD, weigh them accurately, add 30 mL of distilled water and stir to dissolve, then add a proportioning amount of carbodiimide (EDC) (Table 1), and stir to dissolve. According to chitosan: stearic acid (molar ratio) 1: 100, take stearic acid and dissolve in 20mL ethanol solution. The above two solutions were mixed, at 400r·min -1 Under the condition of magnetic stirring, the reaction was carried out at a constant temperature of 80°C for 5h, and after 5h, the room temperature (25°C) was maintained, and the stirring was continued for 6h until the reaction solution was clear. The final reaction solution was placed in a dialysis bag and dialyzed with distilled water for 48 hours. After the dialysate was freeze-dried, the residual stearic acid was washed with absolute ethanol to obtain chitooligosacchar...
Embodiment 2
[0037] Embodiment 2: the synthetic method of the 2nd kind chitosan-fatty acid graft:
[0038] Take 0.4 g of chitosan oligosaccharides with an average molecular weight of 19 kD, accurately weigh, add 30 mL of distilled water and stir to dissolve, add 1.2 g of carbodiimide, and stir to dissolve. According to chitosan: fatty acid (molar ratio) 1: 20, weigh fatty acid (myristic acid, palmitic acid, stearic acid, behenic acid any one of them) respectively, dissolve in 20mL ethanol solution. The above two solutions were mixed, at 400r·min -1 Under the condition of magnetic stirring, the reaction was carried out at a constant temperature of 80°C for 5h, and after 5h, the room temperature (25°C) was maintained, and the stirring was continued for 6h until the reaction solution was clear. The final reaction solution was placed in a dialysis bag and dialyzed with distilled water for 48 hours. After the dialysate was freeze-dried, the residual fatty acid was washed with absolute ethanol...
Embodiment 3
[0042] Embodiment 3: the synthesis of the 3rd kind of chitosan-fatty acid graft:
[0043] Take 0.4 g of chitosan oligosaccharides with an average molecular weight of 19 kD, weigh them accurately, add 30 mL of distilled water and stir to dissolve, then add a proportioning amount of carbodiimide (Table 3), and stir to dissolve. According to chitosan: fatty acid (molar ratio) 1:1, 1:10, 1:20, 1:50, 1:100, stearic acid was weighed and dissolved in 20mL ethanol solution. The above two solutions were mixed, at 400r·min -1 Under the condition of magnetic stirring, the reaction was carried out at a constant temperature of 80°C for 5h, and after 5h, the room temperature (25°C) was maintained, and the stirring was continued for 6h until the reaction solution was clear. The final reaction solution was placed in a dialysis bag and dialyzed with distilled water for 48 hours. After the dialysate was freeze-dried, the residual stearic acid was washed with absolute ethanol to obtain oligoch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com