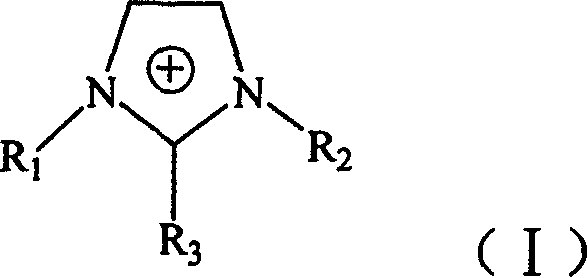
Amino group-containing chiral ionic liquid and its prepn process and application
A chiral ionic liquid and amino group-containing technology, applied in the field of chiral ionic liquids, can solve the problems of difficult recovery of catalyst, decrease in yield and ee value, etc., and achieve excellent chiral catalytic performance, good steric hindrance, and convenience. Repeated effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of 1-[(2S)-2-aminopropyl]-3-hexylimidazolium bromide
[0053] Add (S)-1-(bromomethyl)ethylamine hydrobromide (22.12g, 0.1mol), N-hexylimidazole (15.51g, 98%, 0.1mol) and ethanol (60mL) into a 100mL three-necked flask, Reflux reaction for 20 h, neutralize and precipitate under reduced pressure, wash with ethyl acetate (2×20 mL), and distill off the solvent to obtain the target ammonium halide salt (27.55 g, yield 95%).
Embodiment 2
[0054] Example 2 Preparation of 1-[(2S)-2-amino-3-methylpentyl]-2-methyl-3-methylimidazolium chloride salt
[0055] Add (S)-1-(chloromethyl)-2-methylbutylamine hydrochloride (17.37g, o.1mol), 1,2-dimethylimidazole (7.84g, 98%, 0.08mol) and acetonitrile (60mL), reflux reaction 40h, decompression precipitation after neutralization, wash (2 * 20mL) with ethyl acetate, obtain target ammonium halide salt (16.67g, yield 90 %).
Embodiment 3
[0056] Example 3 Preparation of 1-[(2S)-2-amino-4-methylhexyl]-3-ethylimidazolium bromide
[0057] Add (S)-1-(bromomethyl)-3-methylpentylamine hydrobromide (26.36g, 0.1mol) and N-ethylimidazole (10.78g, 98%, 0.11mol) into a 100mL three-necked flask and ethanol (60mL), reflux reaction for 24h, neutralization, precipitation under reduced pressure, washing with ethyl acetate (2×20mL), and distillation to remove the solvent to obtain the target ammonium halide salt (26.50g, yield 96%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com