Fowl vaccine immunopotentiator of chicken interleuken-18, and its application
A technology of interleukin and immune enhancer, applied in antiviral agents, peptide/protein components, medical preparations containing active ingredients, etc., can solve the problems of incomplete control of infectious diseases, frequent occurrence of immune failure, and limited use range and other issues, to achieve the effect of prolonging immunity, facilitating popularization and application, and enhancing resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment describes the method for obtaining chicken interleukin-18 (IL-18) gene provided by the present invention, comprising the following steps:
[0028] (1) Preparation of Chicken Spleen Lymphocyte Suspension Aseptically isolate the fresh spleen of 14-day-old Zhejiang breed Xiaoshan chick, grind it with a tissue grinder and add Ca-free 2+ , Mg 2+ Hank's solution, washed twice by centrifugation at 1300g for 20min, and then washed twice with RPMI1640 culture medium. After staining with trypan blue (1% Trypan blue) and counting viable cells, the cells were diluted into a single-cell suspension of a certain concentration with RPMI1640 (containing 10% calf serum, 1% penicillin and 1% streptomycin) culture medium.
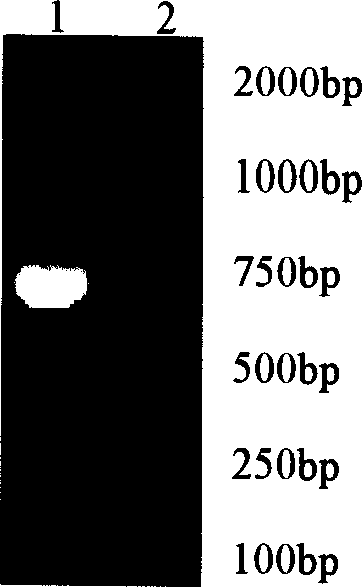
[0029] (2) Synthesis of chicken IL-18cDNA by RT-PCR Culture spleen lymphocytes according to the optimal condition of IL-18 induced in vitro by screening, respectively stimulated by LPS with a final concentration of 10 μg / ml for 5h, 10h, 24h, and 48h to ...
Embodiment 2
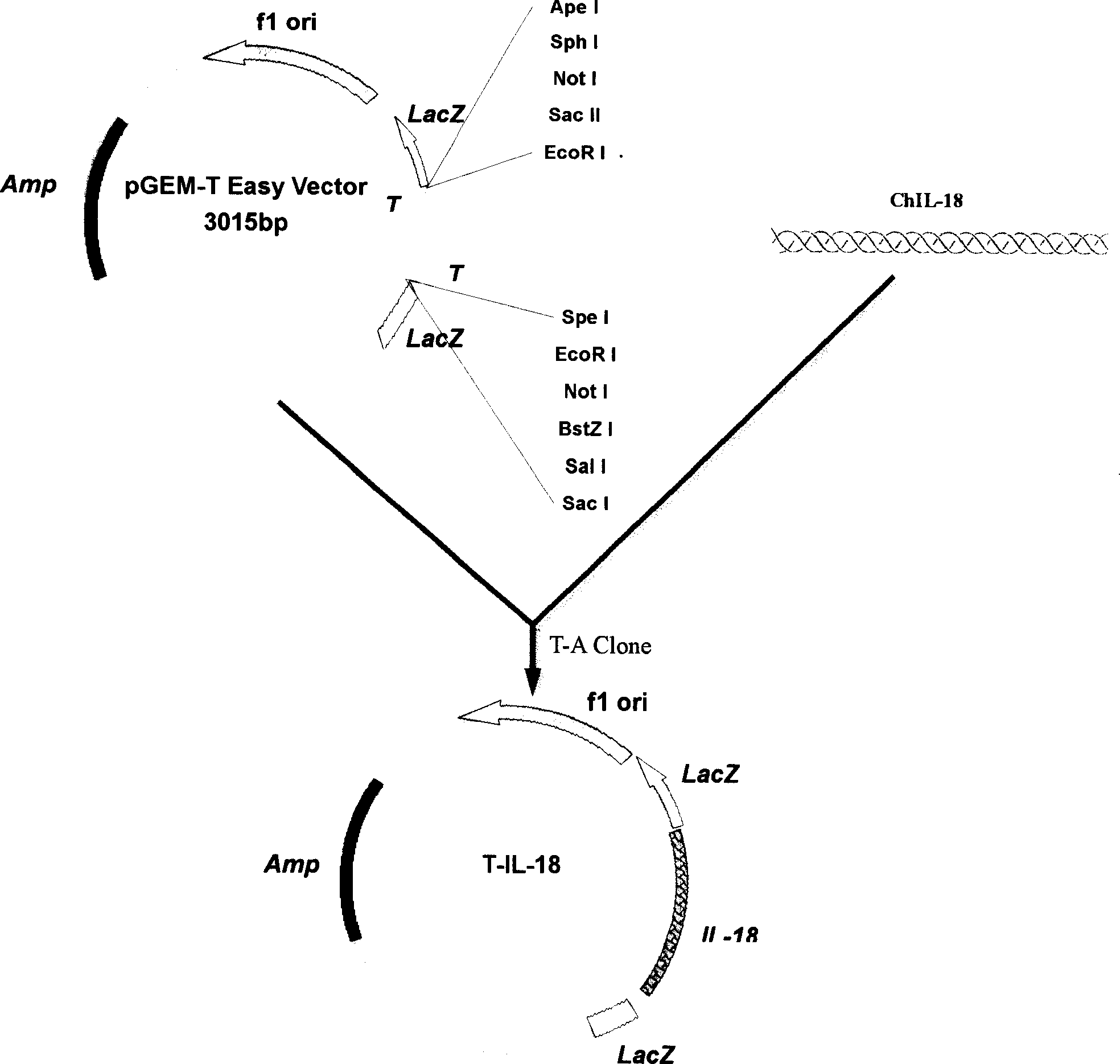
[0037] The present embodiment has described the method for obtaining chicken interleukin 18 (IL-18) gene eukaryotic expression plasmid pCI-IL-18, and its steps include:
[0038] (1) Amplification of chicken IL-18 cDNA Primers were redesigned at both ends of the above-mentioned chicken IL-18 gene (591 bp in total, encoding 196 amino acids)
[0039] P3 (5'-CG GAATTC ATGAGCTGTGAAGAGATCG-3')
[0040] P4 (5'-CC GTC GAC TCATAGGTTGTGCCTTTCA-3')
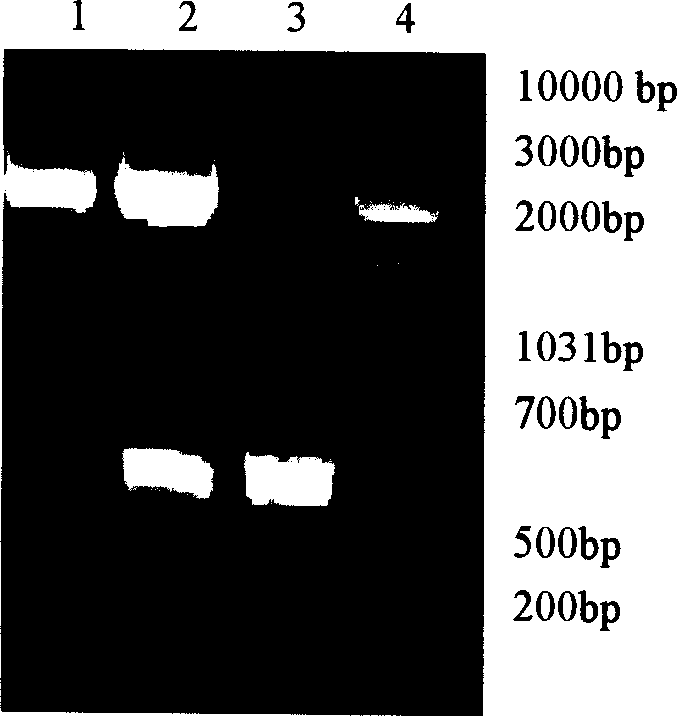
[0041] Both ends of the primers were respectively introduced with enzyme cutting sites EcoR I (underlined at P3) and SalI (underlined at P4), and the primers were synthesized at Shanghai Boya Biotechnology Co., Ltd. Using T-IL-18 as a template and P3 and P4 as primers, the cDNA of chicken IL-18 was amplified according to the following PCR cycle program: pre-denaturation at 94°C for 2 min, denaturation at 94°C for 45 s, annealing at 55°C for 1 min, extension at 72°C for 45 s, Extend at 72°C for 10 min, a total of 30 cycles. PCR product...
Embodiment 3
[0053] This example describes the method for obtaining recombinant chicken interleukin 18 (IL-18) protein. Include the following steps:
[0054] (1) Expression of Chicken Interleukin 18 (IL-18) Gene Eukaryotic Expression Plasmid pCI-IL-18 in Mammalian Vero Cells
[0055] The operation steps of liposome-mediated expression of chicken pCI-IL-18 in mammalian Vero cells are as follows:
[0056] Inoculate 2 × 10 on a 6-well cell culture plate 5 1 cell in 2ml medium containing fetal bovine serum; 37°C 5% CO 2 Cultivate in an incubator for 18-24h to 40-60% cell confluency: Prepare liquid in an Eppendorf tube: Slolution A: Dilute 8 μg DNA to 100 μl serum-free OPTI-MEM I Reduced Serum Medium medium (available from Gibco Company ); Solution B: Dilute 10 μl Lipofectin Reagent (available from Life Technology Company) into 100 μl serum-free OPTI-MEM IReduced Serum Medium medium. In order to achieve the best transfection effect, DNA should not exceed 20μg / ml and Lipofectin Reagent shoul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com