Medicine for treating endometriosis and its prepn
A technology for endometriosis and ectopic disease, which can be used in pharmaceutical formulations, drug combinations, drug delivery, etc., and can solve the problems of difficult swallowing by patients and difficulty in swallowing the total amount of granules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] The preparation of embodiment 1 dispersible tablet
[0103] Ingredients: Megestrol Acetate 100g
[0104] Sodium starch glycolate 23.8g
[0105] Crospovidone 17g
[0106] Pregelatinized starch 17g,
[0107] Microcrystalline Cellulose 178.8g
[0108] Silica 3.4g,
[0109] 5% polyvinylpyrrolidone ethanol solution appropriate amount
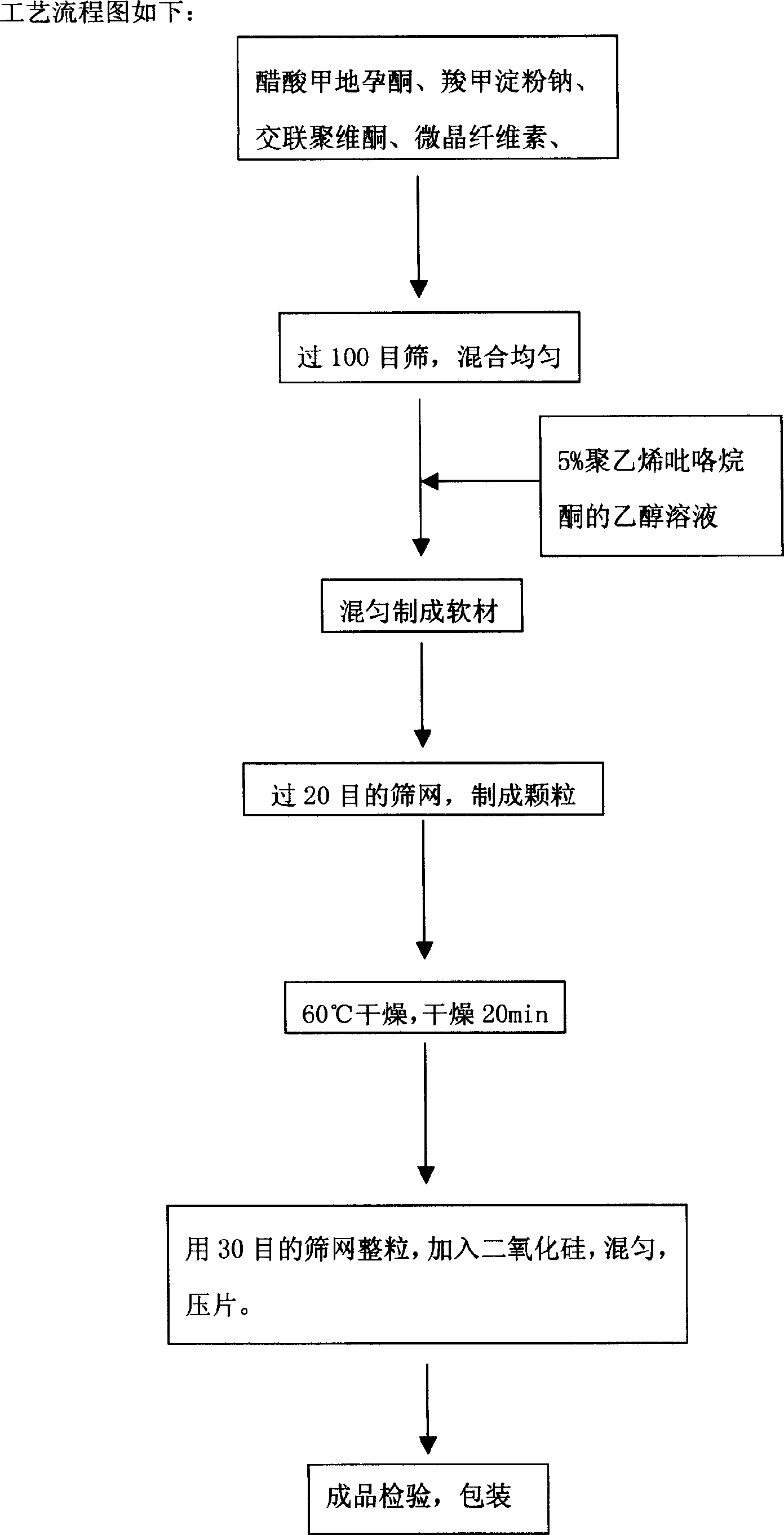
[0110] Take by weighing medroxyprogesterone acetate, carboxymethyl starch sodium, crospovidone, pregelatinized starch, and microcrystalline cellulose of the prescribed amount, and pass through a 100-mesh sieve to mix them evenly; add an appropriate amount of 5 Mix the ethanol solution of % polyvinylpyrrolidone to make a soft material; press the soft material through a 20-mesh sieve to make granules; after the wet granules are made, immediately put them in an oven to dry at 60°C, and take them out after drying for 20 minutes; use 30 The purpose sieve is sized, add silicon dioxide, and mix well. Tablet;...
Embodiment 2
[0111] Embodiment 2, the preparation of capsule
[0112] Ingredients: Megestrol Acetate 100g
[0113] Sodium starch glycolate 23.8g
[0114] Crospovidone 17g
[0115] Pregelatinized starch 17g,
[0116] Microcrystalline Cellulose 178.8g
[0117] Magnesium Stearate Appropriate amount
[0118] 5% polyvinylpyrrolidone ethanol solution appropriate amount
[0119] Take by weighing medroxyprogesterone acetate, carboxymethyl starch sodium, crospovidone, pregelatinized starch, and microcrystalline cellulose of the prescribed amount, and pass through a 100-mesh sieve to mix them evenly; add an appropriate amount of 5 Mix the ethanol solution of % polyvinylpyrrolidone to make a soft material; press the soft material through a 20-mesh sieve to make granules; after the wet granules are made, immediately put them in an oven to dry at 60°C, and take them out after drying for 20 minutes; use 30 Purpose Sieve the grains, mix them evenly, divide them i...
Embodiment 3
[0120]Example 3 Preparation of Granules
[0121] Ingredients: Megestrol Acetate 100g
[0122] Appropriate amount of sucrose
[0123] Proper amount of hypromellose
[0124] Other Appropriate amount
[0125] Weigh the prescribed amount of raw materials medroxyprogesterone acetate and other auxiliary materials through a 100-mesh sieve, prepare hypromellose with pure water to a suitable concentration as an adhesive, mix the remaining raw materials and auxiliary materials evenly, and use the prepared Adhesive granulation, drying at 60-65°C, granulation, sieving, packaging; finished product inspection, packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com