No-adjuvant therapeutic protein vaccine containing heat shock protein and HPV 16Z protein antigen
A technology of heat shock protein and protein antigen, applied in the field of unadjuvanted therapeutic protein vaccine containing heat shock protein and HPV16Z protein antigen, which can solve problems such as lack of adjuvant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The construction of embodiment one Hsp65-E6 / E7 fusion protein expression plasmid and bacterial strain
[0017] 1.1 Synthesis of E6 / E7 fusion gene
[0018] Using the whole genome sequence of the HPV16Z virus strain cloned from Shanxi by Professor Zhang Wei as a template, the E6 and E7 fragments were obtained by PCR, and on this basis, 3 pairs of primers were designed to spot the 24, 26, 58, and 91 codons of E7 Mutate, and at the same time PCR amplify the codon of 88 amino acids at the C-terminus of E6, and fuse the obtained part of the E6 fragment with the mutated E7 fragment.
[0019] 1.2 Acquisition of PET28a-Hsp65
[0020] Purchase 2 tubes of BCG from the China Institute of Biological Products, use STE to suspend the bacteria, centrifuge and resuspend in 400μl 0.05MTris-HCl (PH 8.5), 0.05M EDTA, 15% sucrose, add 0.4mg lysozyme, 37℃ Act for 30 minutes, then add 2% proteinase K to a final concentration of 0.1mg / ml, act for 10 minutes at 25°C, add 4mg SD...
Embodiment 2
[0023] Example 2 Identification of fusion protein expression products and optimization of expression conditions
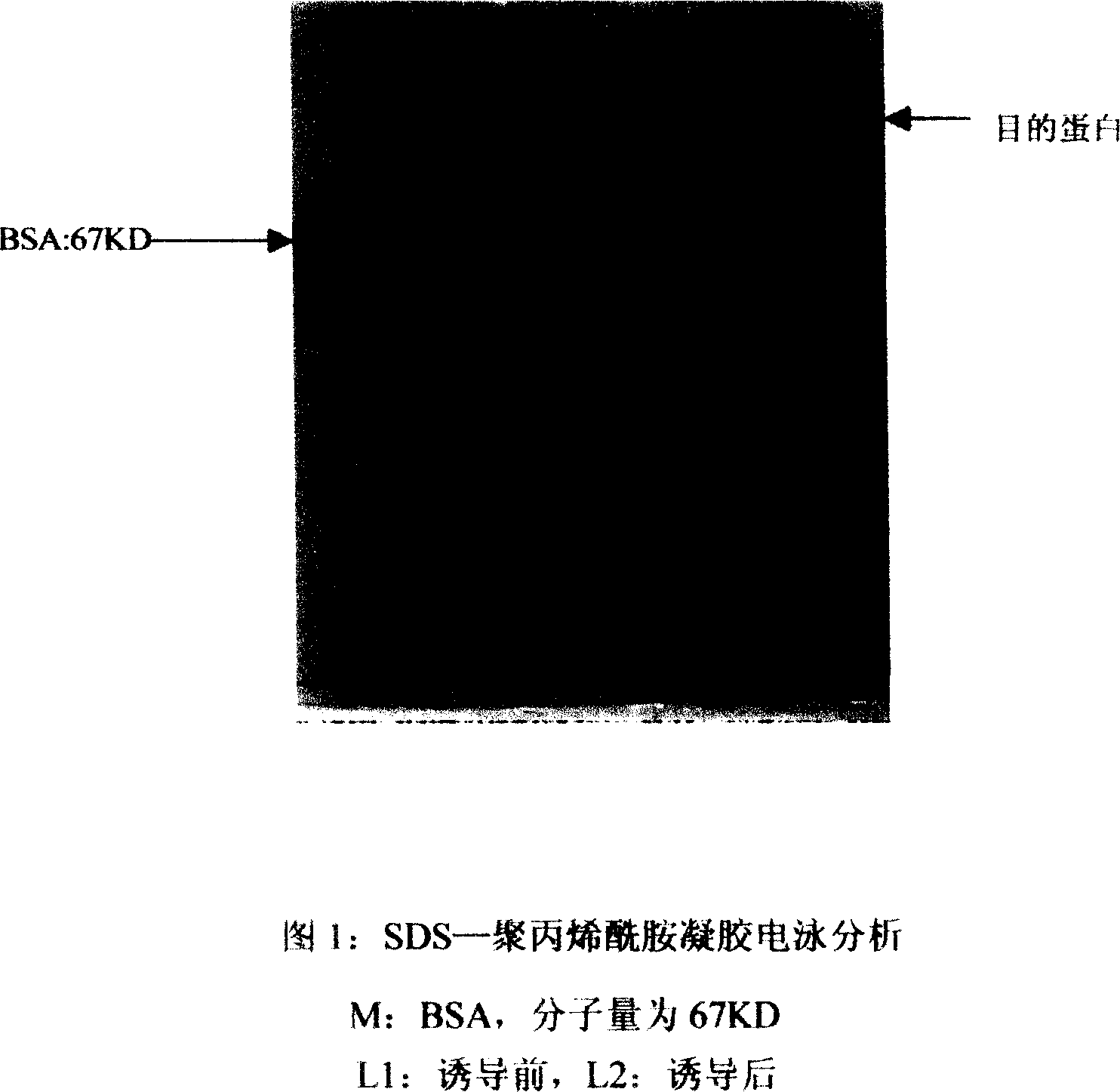
[0024] When the Escherichia coli BL21 (DE3) transformed with the expression plasmid was grown at 37°C in a medium containing kanamycin until the OD600 was between 0.6-1.0, part of the bacterial liquid was taken out, and the rest was added with IPTG to a medium concentration of 1 mmol / L, continue to cultivate at 37°C for 3 hours, take 1ml of the induced and uninduced bacterial solutions, collect the bacterial cells by centrifugation, add the loading buffer in a water bath at 100°C for 5 minutes, centrifuge to get the supernatant and directly perform SDS-PAGE electrophoresis, and also Carry out electrophoresis of protein standard BSA. After electrophoresis, Coomassie brilliant blue staining for more than 2 hours, and then decolorization treatment with decolorization solution, the decolorization process can be accelerated by heating. Compared with BSA, the...
Embodiment 3
[0026] Example 3 Fermentative expression and purification of fusion protein
[0027] After recovering the preserved expression strains, culture them overnight in 10ml LB medium at 37°C with shaking at 150 rpm, inoculate 5ml into 500ml LB medium, and incubate for 24 hours. Carry out fermentation culture in 9.5L TB culture medium, adjust pH value to 6.8, 300 rev / min, culture 4 hours, guarantee that dissolved oxygen is above 50% during this period. Add IPTG with a final concentration of 1 mmol / L, induce for 3 hours, harvest the bacterial liquid, collect the bacterial cells by centrifugation, and suspend them in the lysis buffer A (10 mM Tris-Hcl, 0.5 mM β- mercaptoethanol, pH8.0, 200ug / ml lysozyme), stored at -70°C for later use. When in use, thaw the frozen bacterial suspension at room temperature, add 2ug / ml protease inhibitor PMSF, sonicate the bacteria, centrifuge at 15000rpm for 10 minutes, collect the precipitate, and wash the precipitate with inclusion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com