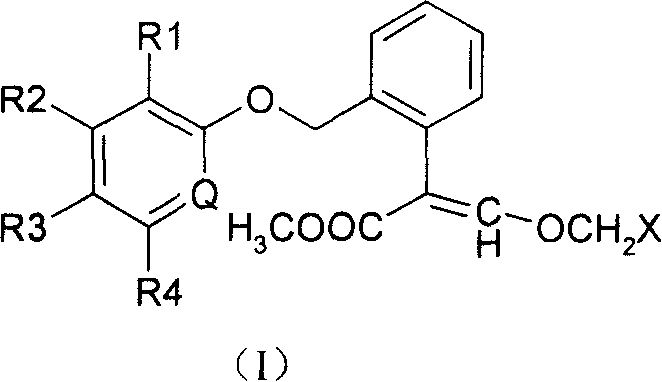
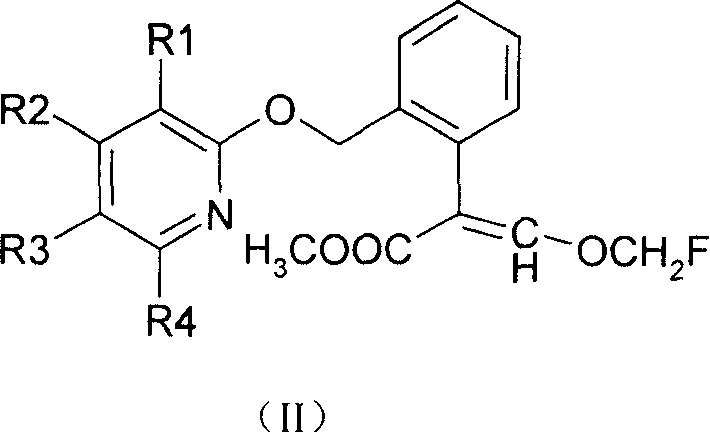
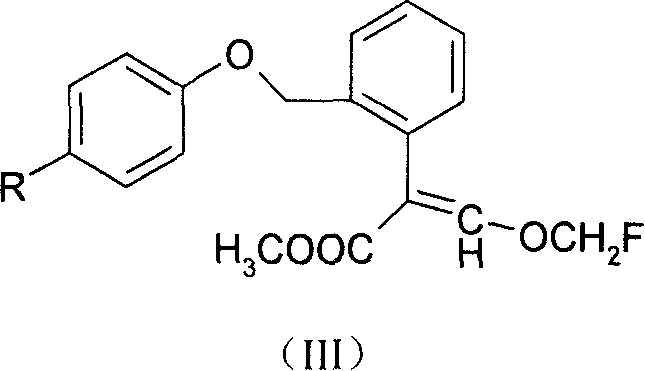
Methoxyl group displacement methyl acrylate compound bactericidal agent
A technology of methyl methoxy acrylate and methyl fluoromethoxy acrylate, which is applied in the application field of agricultural fungicides and can solve the problem of no side chain, no involvement, no side chain benzene ring nitro or alkoxycarbonyl and other problems, to achieve the effect of reasonable toxicity, high-efficiency bactericidal activity, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Compound 1
[0031] Synthesis of 2-[2-(5-cyano-2-methyl-phenyloxymethyl)-phenyl]-3-methoxymethyl acrylate
[0032] At room temperature, 1.33g (0.01 mole) of 2-methyl-5-cyanophenol was added to a three-necked flask containing 60ml of dry acetone in 150ml, then 2.76g (0.02 mole) of potassium carbonate was added, stirred at room temperature for 30 minutes, and then 2.85 g (0.01 mol) of methyl 2-(2-(bromomethyl)phenyl)-3-methoxyacrylate was added slowly. Then the reflux reaction was completed for 4 hours, filtered and concentrated to obtain a crude product. Column chromatography was performed using a mixture of ethyl acetate and petroleum ether (1:4) as the eluent to obtain 3.13 g of compound I as a white solid with a yield of 93%. The product was confirmed by nuclear magnetic resonance (1HNMR) and mass spectrometry (MS) analysis. The analysis results are as follows:
[0033] 1 HNMR: 2.324(3H, s), 3.732(3H, s), 3.885(3H, s), 5.012(2H, s), 6.917-6.920(1H, s), ...
Embodiment 2
[0036] Example 2 Compound 4
[0037] Synthesis of 3-[2-(2-methoxy-1-methoxycarbonyl-vinyl)-phenylmethoxy]-4-methyl-benzoic acid methyl ester
[0038] At room temperature, 3.32g (0.02 moles) of methyl 3-hydroxy-4-methylbenzoate was added to a 250ml three-necked flask filled with 100ml of dry acetone, then 5.52g (0.04 moles) of potassium carbonate was added, and stirred at room temperature for 30 minutes , and then slowly added 5.7 g (0.02 mol) of methyl 2-(2-(bromomethyl)phenyl)-3-methoxyacrylate. Then the reflux reaction was completed for 4 hours, filtered and concentrated to obtain a crude product. Column chromatography was performed using a mixture of ethyl acetate and petroleum ether (1:4) as the eluent to obtain 6.22 g of compound I as a pale yellow solid with a yield of 84%. Melting point: 97.2-101.6°C. The product was confirmed by nuclear magnetic resonance (1HNMR) and mass spectrometry (MS) analysis. The analysis results are as follows:
[0039] 1 HNMR: 2.316 (3H,...
Embodiment 3
[0040] Example 3 Compound 5
[0041] Synthesis of 2-{2-[4-(ethoxyiminomethyl)-phenyloxymethyl]-phenyl}-3-fluoromethoxymethyl acrylate
[0042] At room temperature, 4.95g (0.03 moles) of 4-(ethoxyimine-methyl)-phenol was added to a three-necked flask containing 100ml of dry acetone in 250ml, then 8.28g (0.06 moles) of potassium carbonate was added, stirred at room temperature for 30 minutes, and then slowly added 9.09 g (0.03 moles) of methyl 2-(2-(bromomethyl)phenyl)-3-fluoromethoxyacrylate. Then the reflux reaction was completed for 4 hours, filtered and concentrated to obtain a crude product. Column chromatography was performed using a mixture of ethyl acetate and petroleum ether (1:4) as the eluent to obtain 8.94 g of compound I as a white solid with a yield of 77%. Melting point: 74.1-76.1°C. The product was confirmed by nuclear magnetic resonance (1HNMR) and mass spectrometry (MS) analysis. The analysis results are as follows:
[0043] 1 HNMR: 1.307 (3H, t), 3.823 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com