Mucosal vaccine adjuvants containing bacterial flegellins as an active component
一种疫苗佐剂、鞭毛蛋白的技术,应用在含有效成分的医用配制品、细菌抗原成分、抗体医疗成分等方向,能够解决不适人类、高肠毒素毒性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Construction of transposon library
[0043] Vibrio vulnificus MO6-24 / O type strain (obtained from J. Glenn Morris, Department of Epidemiology, University of Maryland Medical College Hospital) and mini-Tn5 lacZ1 strain including E.coli SM10λpir (from Braunschweig, GBF National Research, Germany) Center for Biotechnology, obtained from Kenneth N. Timmis) were cultured in a shaker at 37°C and 210 revolutions per minute. The two bacteria were cultured in 10 ml of 2.5HI (2.5% NaCl heart infusion) broth and 20 ml of A single colony was inoculated in LB broth (containing 100 μg / ml ampicillin and 100 μg / ml kanamycin).
[0044] The inoculated culture was centrifuged the next day, washed and centrifuged twice with antibiotic-free LB broth medium, and then suspended in 100 microliters of new LB broth medium. A single bacterial suspension of E. coli and Vibrio vulnificus was mixed together and dropped on an LB agar plate. After culturing overnight at 37°C, 800 microliters of ...
Embodiment 2
[0046] Example 2 Screening of Transposon Mutant Colonies Losing Motility
[0047] Each clone of the Vibrio vulnificus MO6-24 / O transposon library prepared in Example 1 was cultured overnight at 37°C, and then inoculated into semi-solid HI containing 0.3% agar using a sterilized toothpick (Heart Extract) agar plate and incubate at 37°C for 6 hours. Then by measuring the range of movement of the bacteria after growth, the degree of motility of the bacteria is determined.
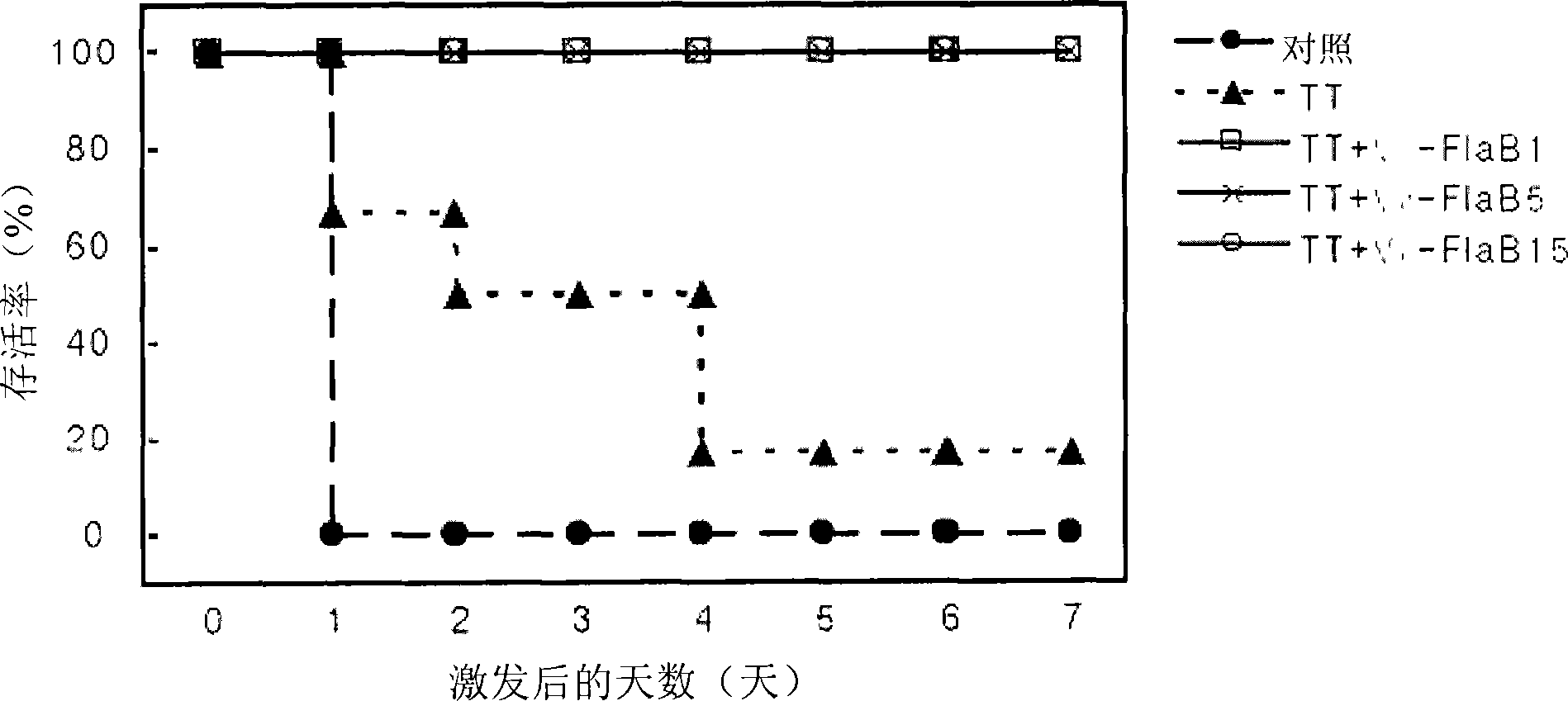
[0048] Three transposon mutant clones that almost completely lost motility were selected through the screening step, and an experiment was performed to identify the mutant genes inserted into the transposon.
Embodiment 3
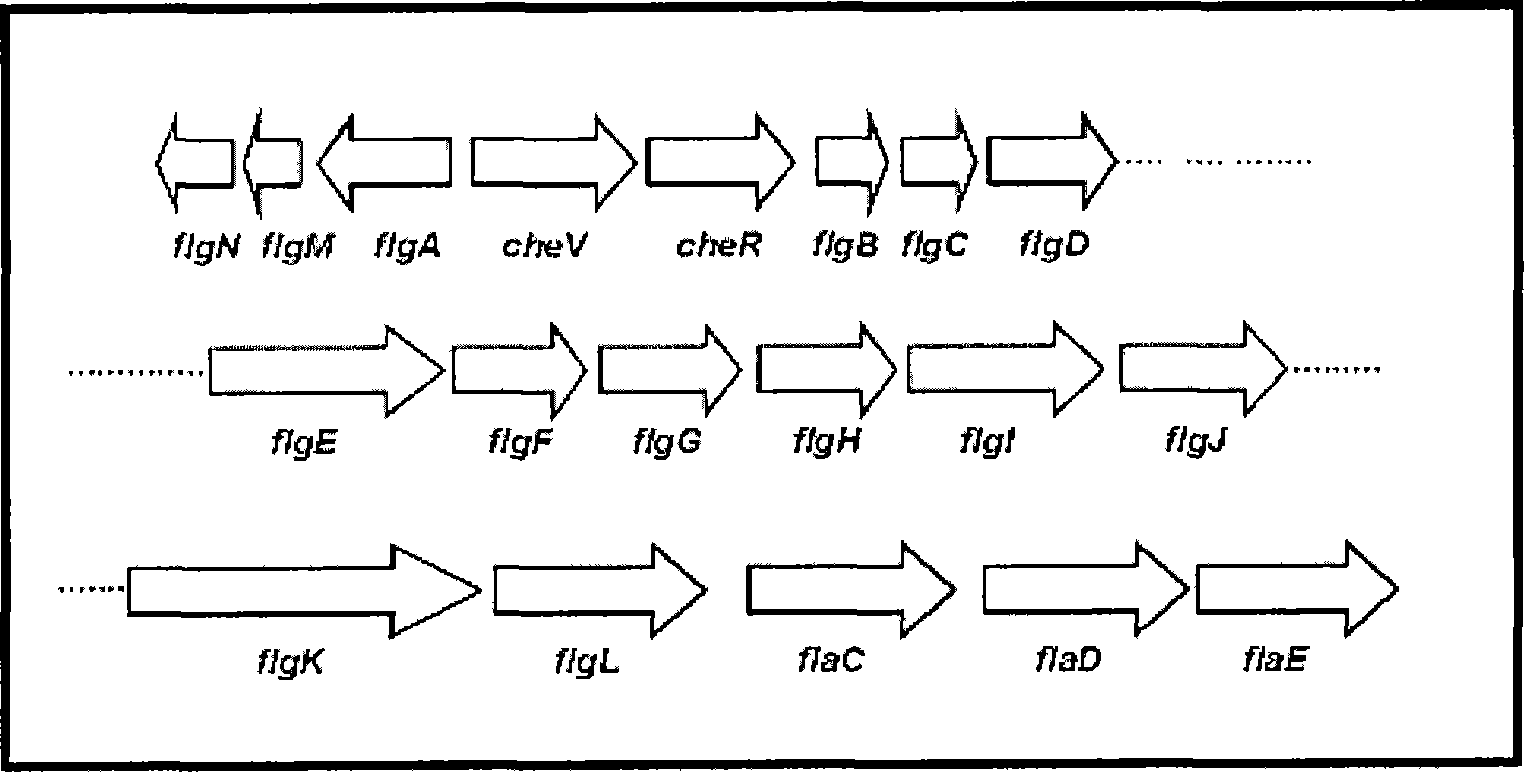
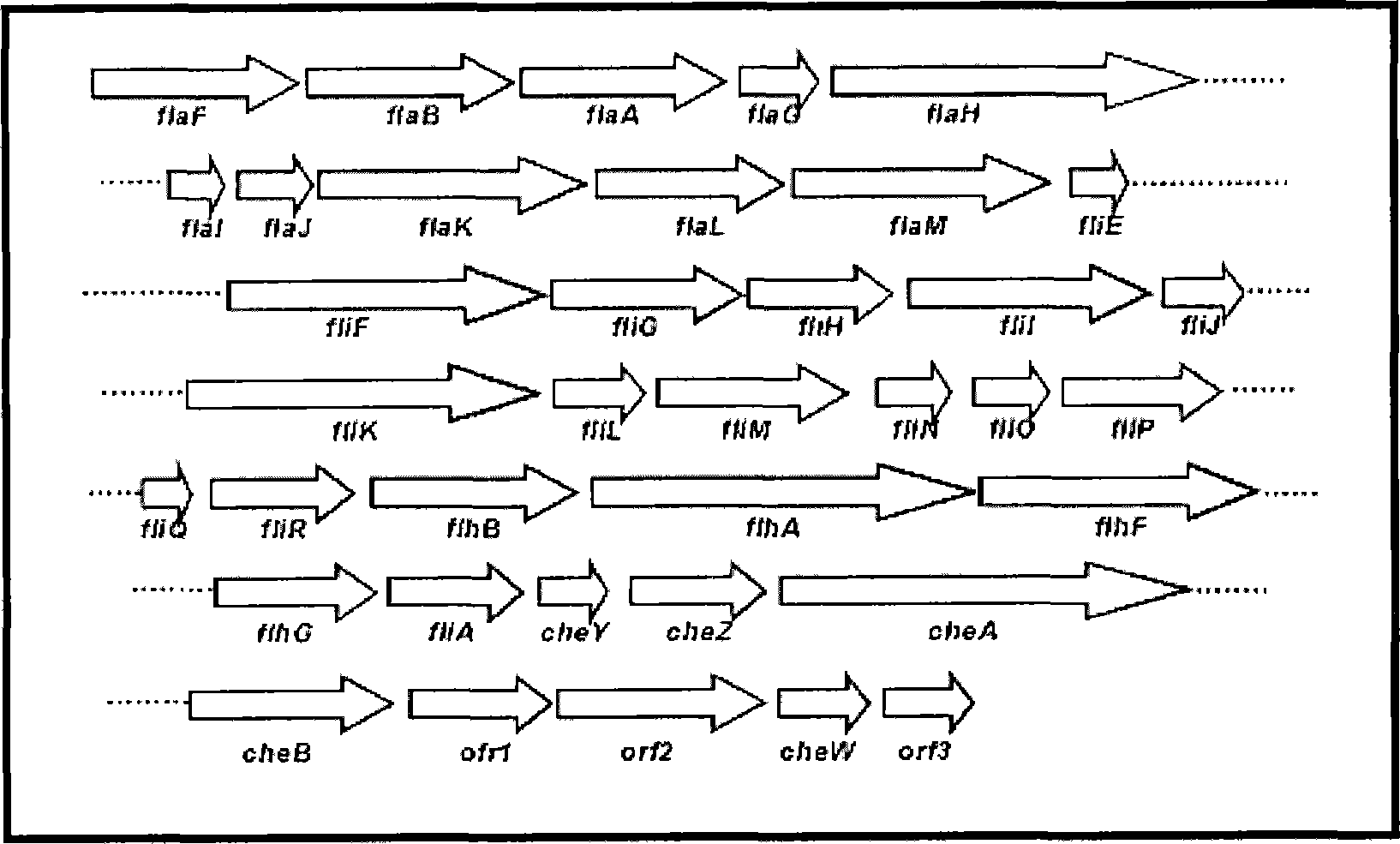
[0049] Example 3 Identification of flagellin operon gene
[0050] The cosmid gene library was screened to clone genes near the transposon insertion region, and DNA fragments were used as primers for random polymerase chain reaction (Polymerase Chain Reaction, PCR) amplification. A two-step PCR amplification method was used to amplify the DNA fragments near the insertion site of the transposon. In the first step of PCR, random primer 1 (5-GGCCACGCGTCGACTAGTCANNNNNNNNNNACGCCC-3) of sequence number 13 and mini-Tn5 lacZ1 specific primer 1 of sequence number 14 (5-TTCTTCACGAGGCAGACCTCAGCGC-3) were used. The first PCR settings are as follows: denaturation at 94°C for 30 seconds, annealing at 30°C for 30 seconds, then extension at 72°C for 1 minute and 30 seconds, 5 cycles; then denaturation at 94°C for 30 seconds, annealing at 45°C for 30 seconds, and extension at 72°C 2 minutes, 30 cycles, and then 30 cycles of PCR reaction. Use the product of the first PCR as a template for the second...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com