Live target adjuvant containing D-galactose and sterol or aliphatic alcohol and its preparation
A technology of galactose and excipients, applied in the field of medicine, can solve the problems of complex reaction and low yield, and achieve the effect of easy degradation and short reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
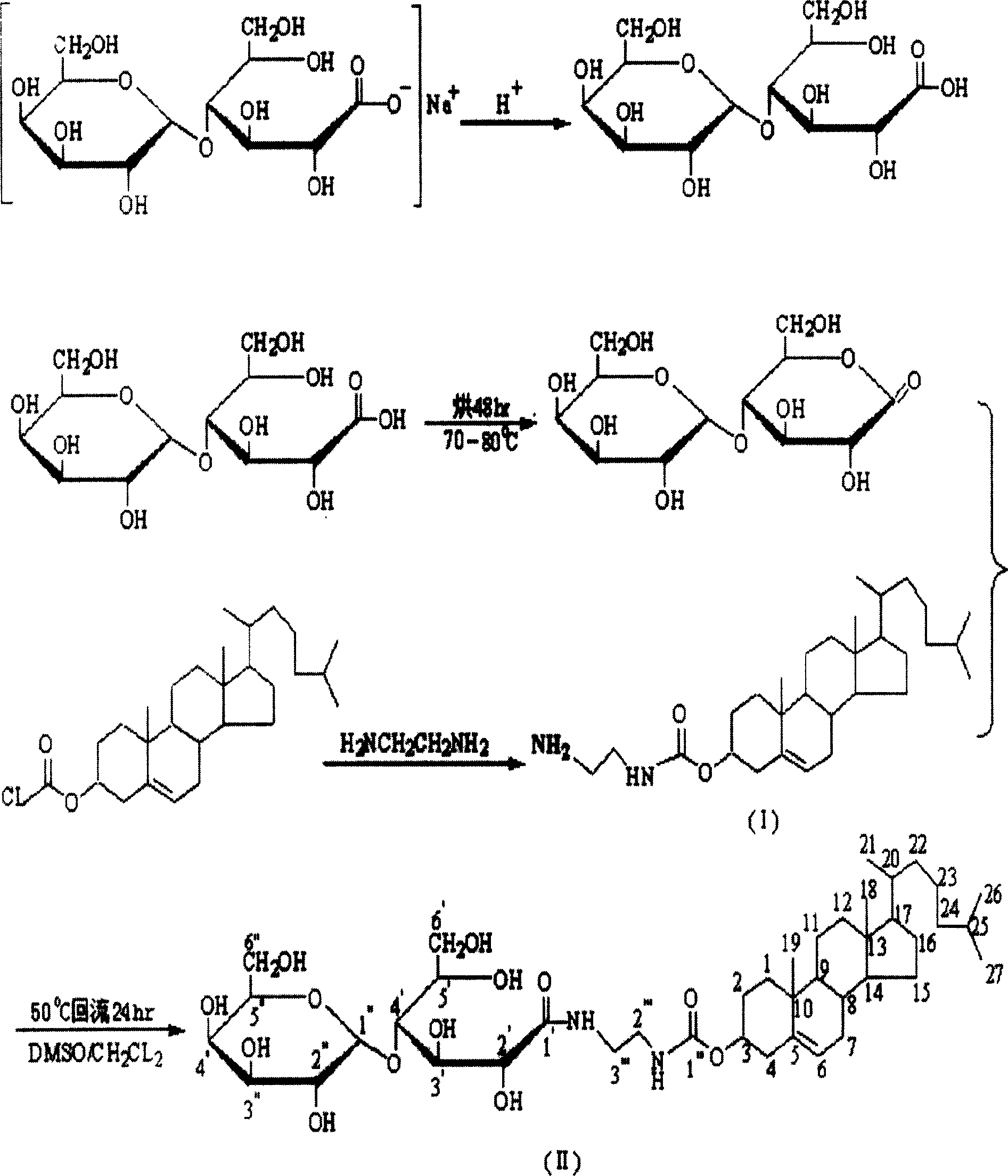
[0035] Embodiment 1: the synthesis of [(2-lactose amido) ethylamino] cholesteryl formate (CH-ED-LA)
[0036] By synthesizing (2-aminoethylamino)cholesteryl formate and then reacting with lactobionolactone to obtain the target compound [(2-lactose amido)ethylamino]cholesteryl formate (CH-ED-LA). See attached figure 1 .
[0037] 1. Synthesis of lactobionolactone
[0038] Take lactobionic acid and add methanol to dissolve, evaporate under reduced pressure at 50°C, remove methanol, repeat 3-4 times, put the solid in an oven at 70-80°C for 48 hours, and obtain lactobionolactone.
[0039] 2. (2-Aminoethylamino) cholesteryl formate (I)
[0040] Take 0.45g cholesteryl chloromethyl ester (1mmol), with 60mL CH 2 Cl 2 Dissolved, placed in the dropping funnel, slowly added to the solution containing 5.84g ethylenediamine (100mmol) CH 2 Cl 2 solution, stirred and reacted in an ice-water bath for 10 hr. Evaporate CH under reduced pressure 2 Cl 2 , add 20mL of water (to wash away t...
Embodiment 2
[0043] Embodiment 2: (2-lactosamido) ethylamino] cholesteryl formate (CH-ED-LA) synthetic
[0044] By synthesizing N-(lactosyloxy) diimide, and then reacting with (2-aminoethylamino) cholesteryl formate to obtain [(2-lactose amido) ethylamino] cholesteryl formate (CH-ED- LA).
[0045] 1. Synthesis of lactobionic acid active ester
[0046] Dissolve 1.80 g of lactobionic acid (5 mmol) in 30 mL of DMF, cool to -15°C to -20°C, add 3.10 g of N,N'-dicyclohexylcarbodiimide (15 mmol), and stir at -15°C for 20 minutes. Rise to -5°C for 15 min, then add 1.15 g of N-hydroxysuccinimide (10 mmol), stir the reaction at -5°C for 1 h, and then stir at room temperature for 25 h. Filter the reaction mixture to remove dicyclohexyl urea, evaporate the obtained filtrate to about 5 mL, add 20 mL of absolute ethanol, add diethyl ether dropwise after ultrasonic dispersion, and precipitate white flocs, then filter with suction, wash with 2 mL of diethyl ether, and dry in vacuo. Obtain white powder ...
Embodiment 3
[0050] Example 3: Synthesis of [(2-lactosamido)hexylamino]cholesteryl formate (CH-HD-LA)
[0051] By synthesizing (2-aminohexylamino) cholesteryl formate and then reacting with lactobionolactone to obtain [(2-lactose amido) hexylamino) cholesteryl formate (CH-HD-LA).
[0052] Take 0.45g cholesteryl chloromethyl ester (1mmol), with 60mL CH 2 Cl 2 Dissolved, placed in the dropping funnel, slowly added to the solution containing 11.6g hexamethylenediamine (100mmol) 2 Cl 2 solution, stirred and reacted in an ice-water bath for 10 hr. Evaporate CH under reduced pressure 2 Cl 2 , add 20mL of water (to wash away the unreacted hexamethylenediamine and its hydrochloride), mix well, and then use CH 2 Cl 2(15ml×2) for extraction, washed with deionized water (15ml×2), and an appropriate amount of anhydrous Na 2 SO 4 Let dry overnight. The solvent was distilled off under reduced pressure to obtain 0.43 g of a gray solid (82.1% yield), TLC (dichloromethane / methanol=9:1); R f = 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com