Method of chemical synthesizing hongjingtian glycoside
A salidroside and chemical synthesis technology, applied in organic chemistry, sugar derivatives, etc., can solve the problems of multiple processes, high cost, and high price, and achieve the effect of shortening the reaction steps and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
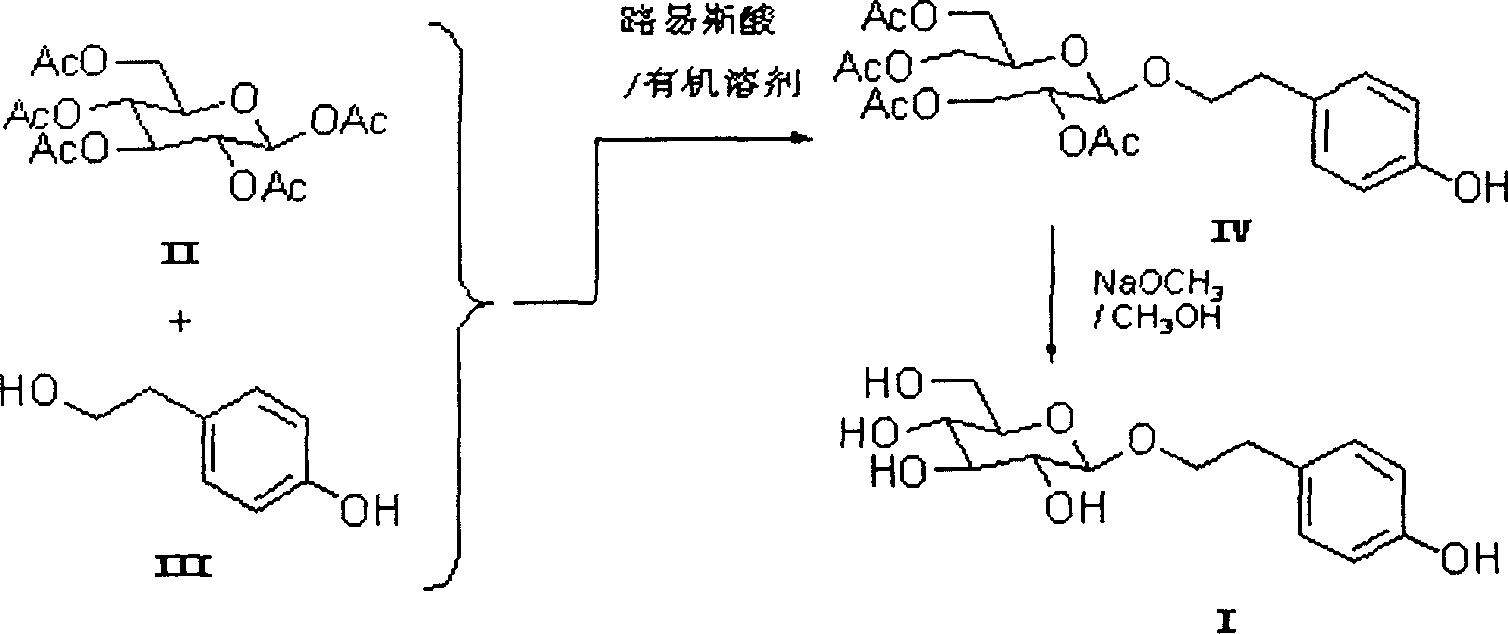
[0021] Add 2 g of 4A molecular sieves to a solution of pentaacetyl-β-D-glucose (2.93 g, 7.5 mmol) in dichloromethane (30 ml), and stir for 1 hour under nitrogen protection. Then, tin tetrachloride (0.585ml, 5.0mmol) was added, immediately after the addition, a suspension of p-hydroxyphenethyl alcohol (0.69g, 5mmol) in dichloromethane (20ml) was added, and the reaction was stirred at room temperature for 2 hours. After the reaction was completed and allowed to stand, pour out the organic layer, then add ethyl acetate to the solid and stir for several minutes, pour out the organic layer, and repeat again. After the organic phases were combined, they were poured into saturated sodium bicarbonate (75ml) under stirring, and after the organic layer was separated, the aqueous layer was extracted with dichloromethane (3 × 15ml), and the organic layers were combined and washed with water (2 × 30ml). Then it was filtered on celite, and the solvent was distilled off under reduced pressur...
Embodiment 2
[0026] Add 2 g of 4A molecular sieves to a solution of pentaacetyl β-D-glucose (2.96 g, 7.5 mmol) in dichloromethane (30 ml), and stir for 1 hour under nitrogen protection. Then, boron trifluoride ether solution (0.63ml, 5.0mmol) was added, and immediately after the addition, a suspension of p-hydroxyphenylethanol (0.69g, 5mmol) in dichloromethane (20ml) was added, and the reaction was stirred at room temperature for 5 hours. 0.30 g of tetraacetyl salidroside was obtained.
[0027] The above-mentioned tetraacetyl salidroside was dissolved in anhydrous methanol (12.5 ml) containing sodium methoxide (0.07 g, 1.3 mmol), and stirred at room temperature for 12 hours. After the reaction was finished, an acidic resin was added to neutralize, and then the resin was filtered off. The filtrate was concentrated under reduced pressure, and separated and purified with a silica gel column to obtain 0.17 g of salidroside, with a total yield of 11%.
Embodiment 3
[0029] Add 2 g of 4A molecular sieves to a solution of pentaacetyl β-D-glucose (3.90 g, 1.0 mmol) in dichloromethane (30 ml), and stir for 1 hour under nitrogen protection. Then, tin tetrachloride (0.88ml, 7.5mmol) was added, immediately after the addition, a suspension of p-hydroxyphenethyl alcohol (0.69g, 5mmol) in dichloromethane (20ml) was added, and the reaction was stirred at room temperature for 2 hours. 0.56 g of tetraacetyl salidroside was obtained.
[0030] The above tetraacetyl salidroside was dissolved in anhydrous methanol (12.5 ml) containing sodium methoxide (0.14 g, 2.5 mmol), and stirred at room temperature for 24 hours. After the reaction was completed, an acidic resin was added for neutralization, and then the resin was filtered off. The filtrate was concentrated under reduced pressure and separated and purified with a silica gel column to obtain 0.33 g of salidroside, with a total yield of 22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com