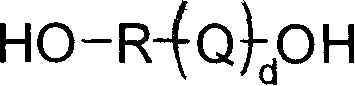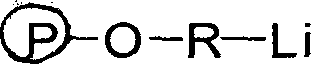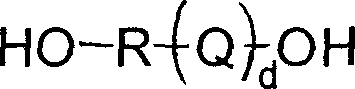Polymer of terminal dihydroxy, and prepartion method
A technology of terminal dihydroxy functional polymers, which is applied in the field of terminal functional polymers, can solve the problems of incomplete conversion of polymer terminal functionalization, etc., and achieve molecular weight control, high yield of functionalization, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 tert-butyldimethylsiloxypropyllithium:
[0041]In a 250ml three-necked flask, 38g (0.4mol) of 3-chloro-1-propanol, 61g (0.4mol) of tert-butyldimethylsilyl chloride, and 80ml of DMF were sequentially added. Then a DMF (30ml) solution containing 30g (0.44mol) of imidazole was added dropwise to the reaction system within 0.5h at 0°C, under N 2 Stir overnight at room temperature under air protection. Add 350ml n-hexane, then 300ml 5% NaHCO 3 The aqueous solution was washed three times, and the n-hexane layer was separated. with anhydrous MgSO 4 After drying, the solvent was evaporated by rotary evaporation, and a colorless liquid was obtained by column separation with a yield of 94%.
[0042] Under Ar atmosphere, add 8g (1.01mol) lithium and 50ml anhydrous cyclohexane to a 250ml three-necked flask equipped with a condenser, a constant pressure dropping funnel, and magnetic stirring. mol) tert-butyl-(3-chloropropoxy)dimethylsilane in cycl...
Embodiment 2
[0043] Example 2 Preparation of tert-butyldimethylsiloxane-2,2-dimethylpropyl lithium
[0044] In a 250ml three-necked flask, 49.0g (0.4mol) of 3-chloro-2,2-dimethyl-1-propanol, 61g (0.4mol) of tert-butyldimethylsilyl chloride, and 100ml of DMF were sequentially added. Then a DMF (30ml) solution containing 30g (0.44mol) of imidazole was added dropwise to the reaction system within 0.5h at 0°C, under N 2 Stir overnight at room temperature under air protection. Add 350ml n-hexane, then 300ml 5% NaHCO 3 The aqueous solution was washed three times, and the n-hexane layer was separated. with anhydrous MgSO 4 After drying, the solvent was evaporated by rotary evaporation, and a colorless liquid was obtained by column separation with a yield of 93%.
[0045] Under Ar atmosphere, add 8g (1.01mol) of lithium and 50ml of anhydrous cyclohexane into a 250ml three-necked flask equipped with a condenser, a constant pressure dropping funnel, and magnetic stirring. mol) tert-butyl-(3-chl...
Embodiment 3 3
[0046] The preparation of embodiment 3 trimethylsiloxyhexyllithium:
[0047] In a 250ml three-necked flask, 48.2g (0.4mol) of 6-chloro-1-hexanol, 43.4g (0.4mol) of trimethylchlorosilane, and 80ml of DMF were sequentially added. Then a DMF (30ml) solution containing 30g (0.44mol) of imidazole was added dropwise to the reaction system within 0.5h at 0°C, under N 2 Stir overnight at room temperature under air protection. Add 350ml n-hexane, then 300ml 5% NaHCO 3 The aqueous solution was washed three times, and the n-hexane layer was separated. with anhydrous MgSO 4 After drying, the solvent was evaporated by rotary evaporation, and a colorless liquid was obtained by separation through a chromatographic column with a yield of 92%.
[0048] Under Ar atmosphere, add 8g (1.01mol) lithium and 50ml anhydrous cyclohexane to a 250ml three-necked flask equipped with a condenser, a constant pressure dropping funnel, and magnetic stirring. mol) cyclohexane (50ml) solution of trimethyl(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com