Multiple apertures releasing osmosis pump and preparation process thereof
一种渗透泵控释、群孔的技术,应用在渗透输送、医药配方、含有效成分的医用配制品等方向,能够解决废品率高渗透泵产品推广、生产难度大、生产周期长等问题,达到提高可靠性和稳定性、劳动强度降低、设备成本降低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Chip composition:
[0037] Venlafaxine Hydrochloride 75g
[0038] Microcrystalline Cellulose 45g
[0039] NaCl 35g
[0041] Coating film composition:
[0042] Cellulose acetate 21g
[0043] Macrogol 6000 9g
[0044] Dibutyl sebacate 2g
[0045] Make 1000 tablets by the following preparation method:
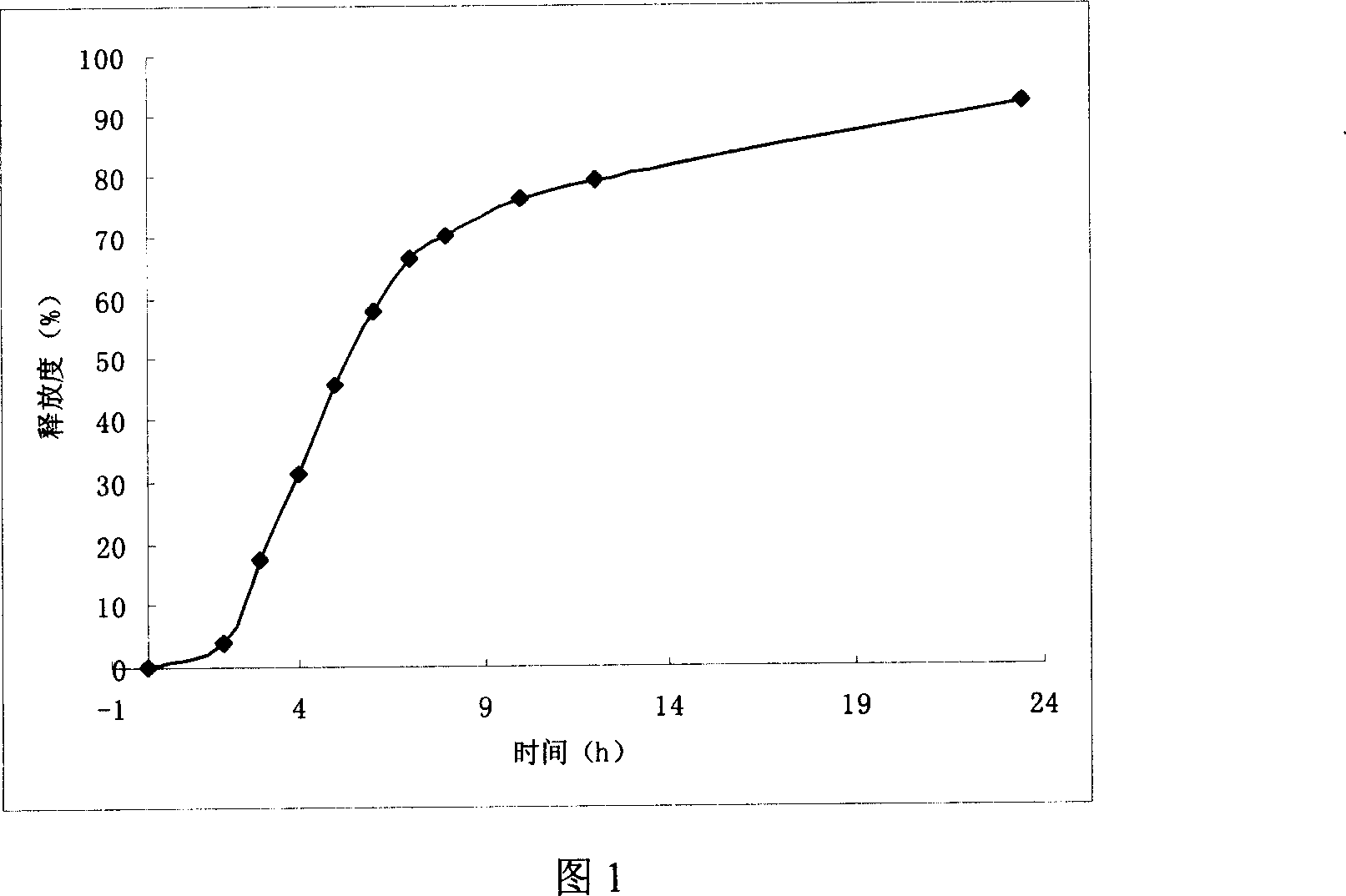
[0046] (1) Tablet core preparation: take sodium chloride and pulverize it, cross a 100 mesh sieve, mix evenly with venlafaxine hydrochloride and microcrystalline cellulose, use ethanol as a wetting agent, make a soft material, cross a 30 mesh sieve for granulation, 45 Dry at ℃ for 2 hours, granulate, add magnesium stearate, mix well, compress into tablets, and compress 1000 tablets by conventional tablet technology.
[0047] (2) Tablet core coating: take cellulose acetate, add 600ml of acetone, stir to dissolve; take another polyethylene glycol, put it in a 50ml measuring bottle, add water to dissolve it, and then add it to the above 1...
Embodiment 2
[0050] Chip composition:
[0051] Venlafaxine Hydrochloride 75g
[0052] Alginic acid 30g
[0053] Propylene Glycol Alginate 20g
[0054] Mannitol 30g
[0056] Coating film composition:
[0057] Polyacrylic resin 5g
[0058] Ethylcellulose 14g
[0059] Hydroxypropyl Cellulose 6g
[0060] Triethyl citrate 2g
[0061] Make 1000 tablets by the following preparation method:
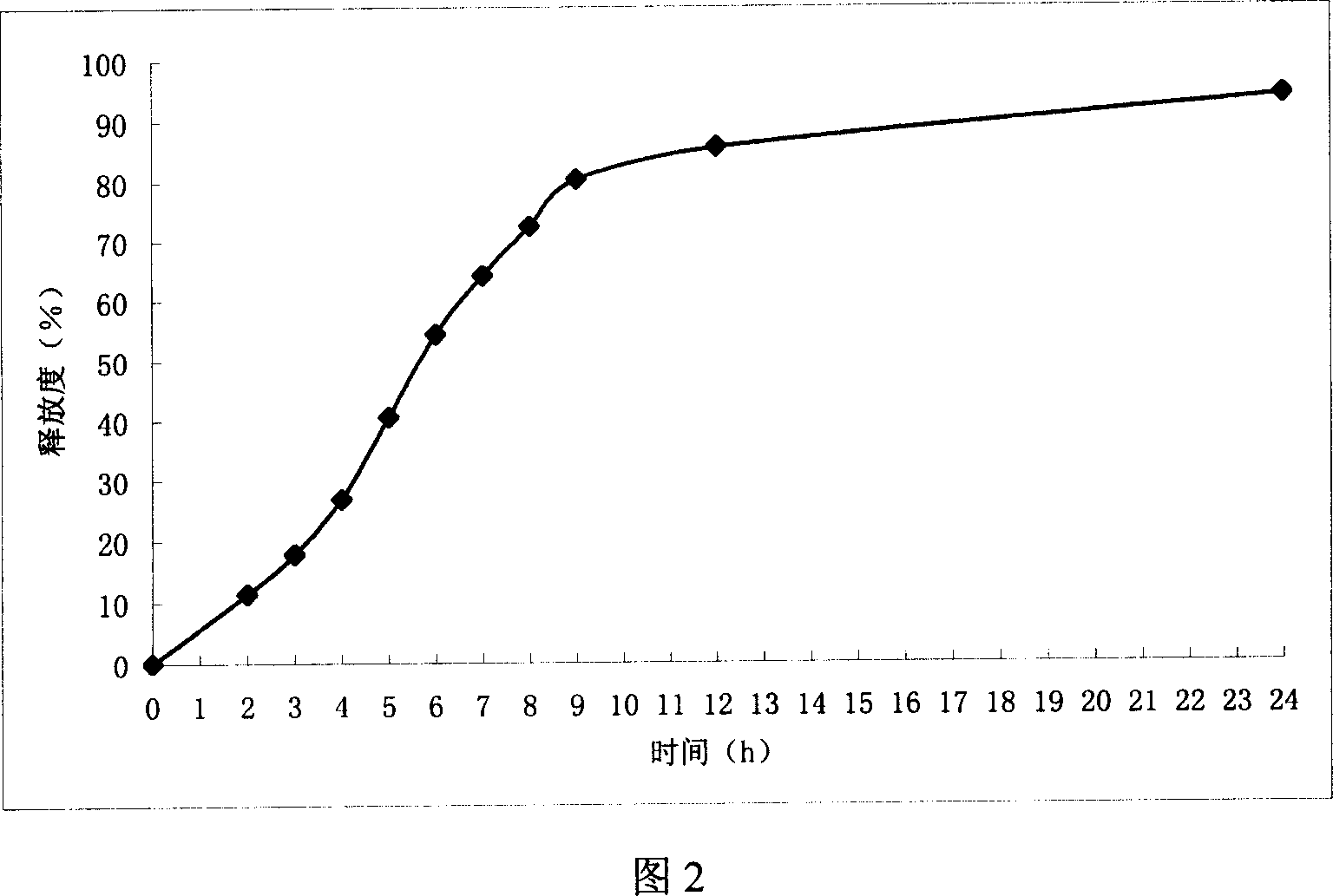
[0062] (1) Tablet core preparation Take mannitol and pulverize it, pass through a 100-mesh sieve, mix evenly with venlafaxine hydrochloride, alginic acid, and propylene glycol alginate, use ethanol as a wetting agent, make a soft material, and pass through a 30-mesh sieve to granulate , dried at 45°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets using conventional tablet technology.
[0063] (2) Tablet core coating: take polyacrylic acid resin, ethyl cellulose, hydroxypropyl cellulose, ad...
Embodiment 3
[0066] Chip composition:
[0067] Venlafaxine Hydrochloride 75g
[0068] Microcrystalline Cellulose 60g
[0070] Magnesium stearate 0.9g
[0071] Coating film composition:
[0072] Cellulose acetate 25g
[0073] Polyethylene glycol (6000) 11g
[0074] Dibutyl sebacate 2.5g
[0075] The composition of the film coating layer containing the main drug:
[0076] Venlafaxine Hydrochloride 10g
[0077] Hypromellose 20g
[0078] Polyethylene glycol (4000) 2g
[0079] Make 1000 tablets by the following preparation method:
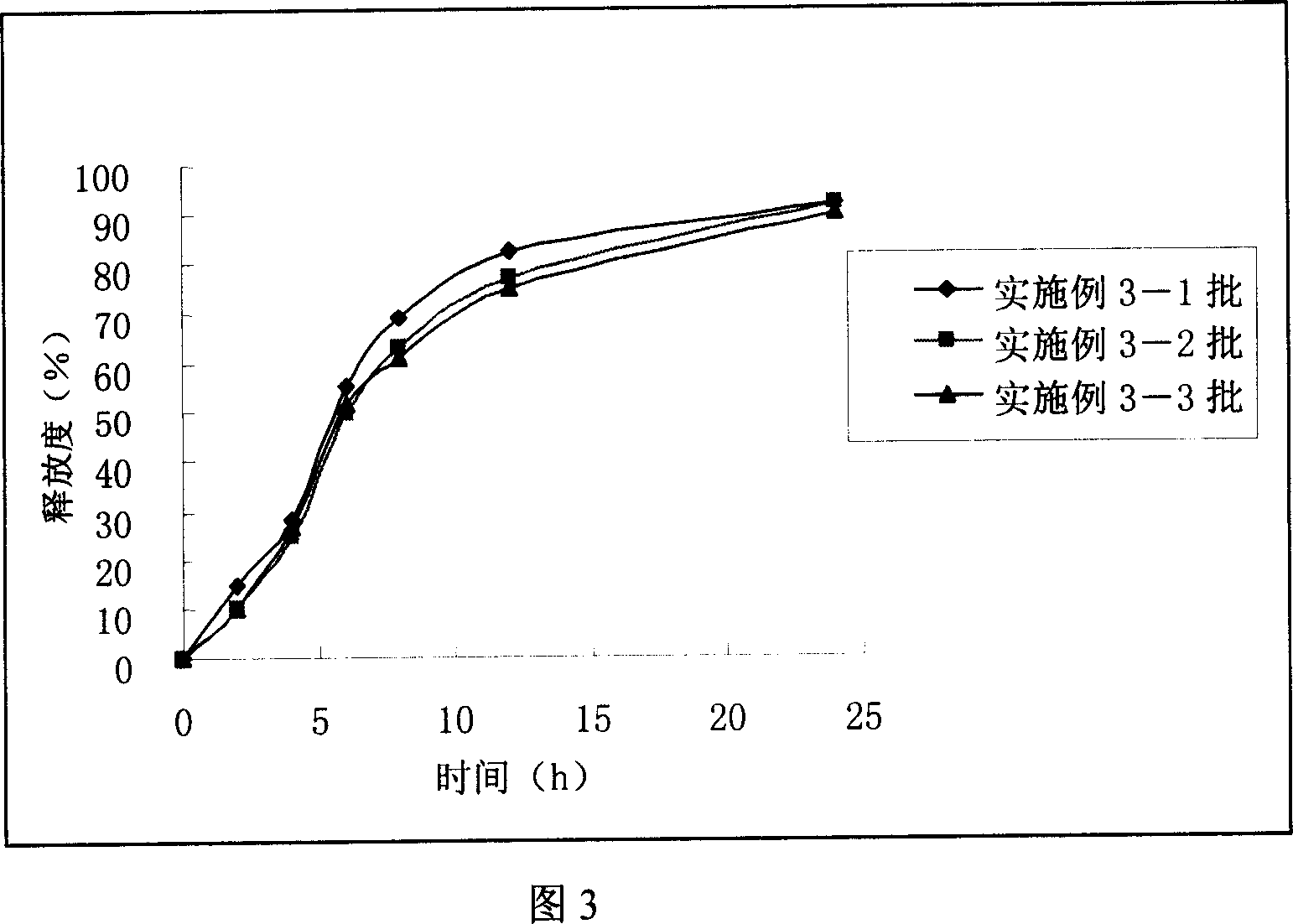
[0080] (1) Tablet core preparation: crush the sodium chloride and set aside. Pass 75g of venlafaxine hydrochloride, 30g of microcrystalline cellulose, and 40g of sodium chloride through a 60-mesh sieve, and mix well. Add 10% polyvinylpyrrolidone k-30 ethanol solution in an appropriate amount to make a soft material, pass through a 30-mesh sieve to granulate, and place it in an oven to heat and dry at 40°C. The prepared granu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com