Lornoxicam composition for injection and preparation process thereof
A technology for lornoxicam and injection, which is applied in the field of lornoxicam injection composition and its preparation, can solve the problems of large side effects, loss of human calcium ions, harm, etc., and achieves wide clinical application, clear and stable solution good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Preparation of injection a
[0077] Lornoxicam 8.6mg
[0078] Arginine 13mg
[0079] Propylene glycol 0.3g
[0080] Sodium dihydrogen phosphate 6mg
[0081] Mannitol 80mg
[0082] Add water for injection to 2ml
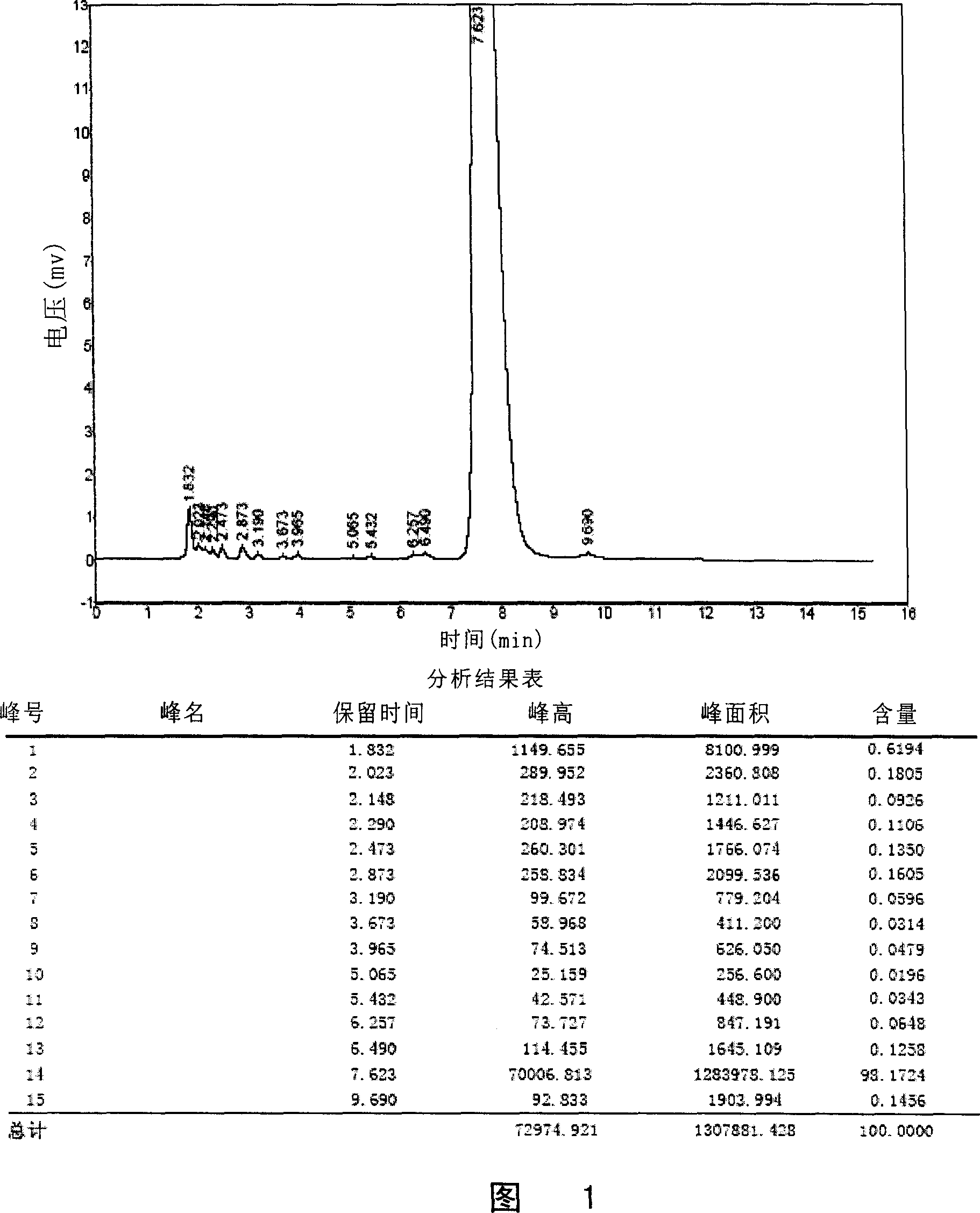
[0083] The lornoxicam solution prepared according to the above prescription was filtered through 0.22 μm. Put the same amount of turbidity standard solution in paired glass tubes for turbidimetry, and observe from the horizontal direction under the fluorescent lamp for comparison. Results The lornoxicam solution was not deeper than No. 1 turbidimetric solution.
[0084] Inject 8 ml of lornoxicam solution prepared as above into 150 ml of sodium chloride injection, as a result, a clear solution is obtained immediately, and the clarity is less than No. 1 turbidimetric solution.
[0085] The results showed that using arginine and propylene glycol as co-solvents of lornoxicam to prepare lornoxicam solution can make the clarity of lorno...
Embodiment 2
[0087] Preparation of injection b
[0088] Lornoxicam 8.6mg
[0089] Arginine 12mg
[0090] Ethanol 0.1g
[0091] Lactic acid amount
[0092] Mannitol 80mg
[0093] Add water for injection to 1ml
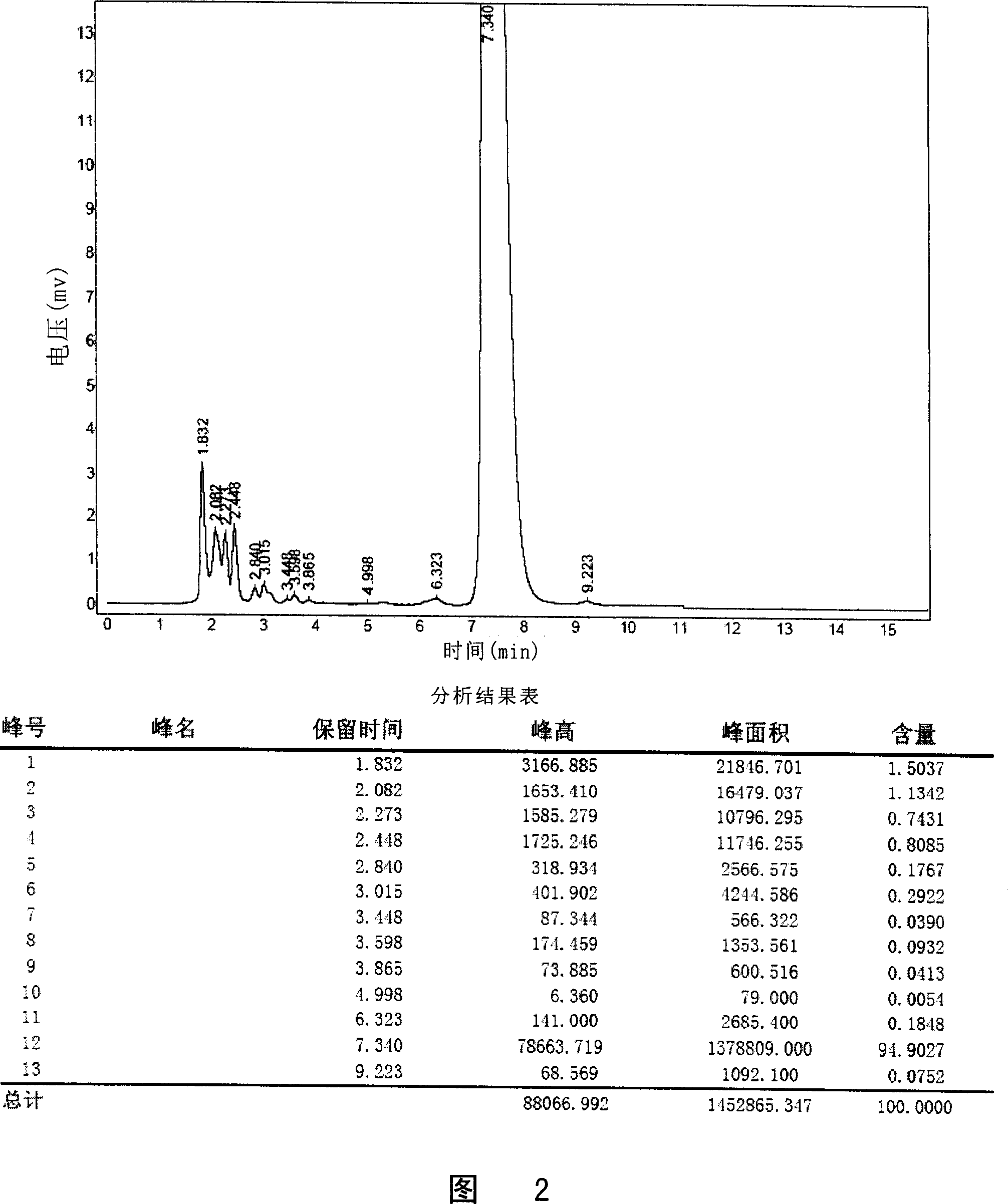
[0094] The lornoxicam solution prepared according to the above prescription was filtered through 0.22 μm. Put the same amount of turbidity standard solution in paired glass tubes for turbidimetry, and observe from the horizontal direction under the fluorescent lamp for comparison. Results The lornoxicam solution was not deeper than No. 1 turbidimetric solution.
[0095] Inject 8 ml of lornoxicam solution prepared as above into 150 ml of sodium chloride injection, as a result, a clear solution is obtained immediately, and the clarity is less than No. 1 turbidimetric solution.
[0096] The results showed that using arginine and ethanol as co-solvents of lornoxicam to prepare lornoxicam solution could make the clarity of lornoxicam solution meet the requi...
Embodiment 3
[0098] Preparation of injection c
[0099] Lornoxicam 8.6mg
[0100] Lysine 6.0mg
[0101] Macrogol 400 0.15g
[0102] Lactic acid amount
[0103] Mannitol 80mg
[0104] Add water for injection to 5ml
[0105] The lornoxicam solution prepared according to the above prescription was filtered through 0.22 μm. Put the same amount of turbidity standard solution in paired glass tubes for turbidimetry, and observe from the horizontal direction under the fluorescent lamp for comparison. Results The lornoxicam solution was not deeper than No. 1 turbidimetric solution.
[0106] Inject 8 ml of lornoxicam solution prepared as above into 150 ml of sodium chloride injection, as a result, a clear solution is obtained immediately, and the clarity is less than No. 1 turbidimetric solution.
[0107] The results showed that using lysine and polyethylene glycol as co-solvents of lornoxicam to prepare lornoxicam solution can make the clarity of lornoxicam solution m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com