Double layer osmotic pump controlled release felodipine medicine composition
An osmotic pump controlled release and dipine double-layer technology, which is applied in drug combinations, sugar-coated pills, pharmaceutical formulations, etc., can solve the problems of unsatisfactory stability and safety of drug release, inability to release drugs, and reduced drug release speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
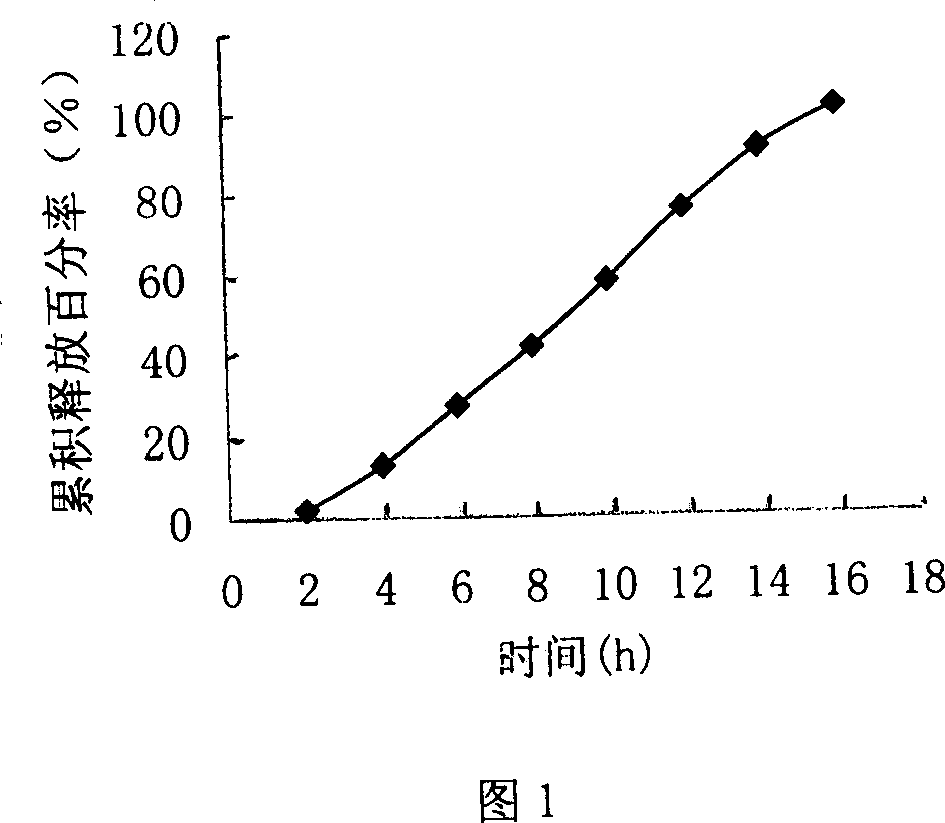
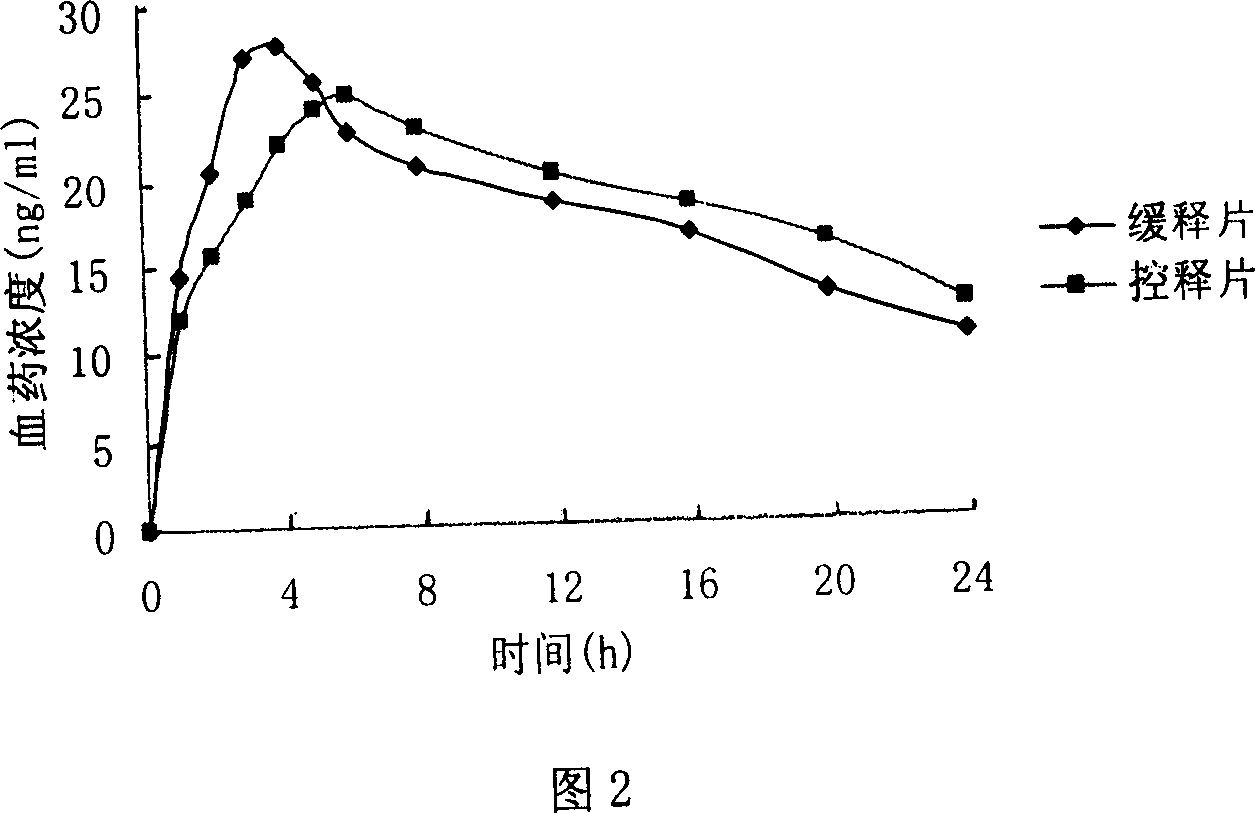
Image
Examples
Embodiment 1
[0077] (1) Drug-containing layer
[0078] Felodipine 2.5g
[0079] Polyoxyethylene 50g
[0080] Magnesium stearate 0.5g
[0081] (2) booster layer
[0082] Polyoxyethylene 20g
[0084] Hypromellose 1g
[0085] Iron oxide red 0.2g
[0086] Magnesium stearate 0.3g
[0087] Makes 1000 pieces
[0088] (3) Prescription of coating solution (1000 tablets dosage)
[0089] Cellulose acetate 10g
[0090] Polyethylene glycol 0.5g
[0091] Proper amount of ethanol
[0092] Preparation:
[0093] 1. Sieve, mix and granulate the raw and auxiliary materials of the drug-containing layer and the booster layer according to the above-mentioned prescription amount, and press the drug-containing layer and the booster layer into a double-layer tablet core by two-time tablet compression technology;
[0094] 2. Coating solution preparation: Add cellulose acetate and polyethylene glycol to an appropriate amount of ethanol solution, stir until completely dissolv...
Embodiment 2
[0098] (1) Drug-containing layer
[0099] Felodipine 10g
[0100] Mannitol 4g
[0101] Polyoxyethylene 120g
[0102] Hypromellose 8g
[0104] 75% ethanol appropriate amount
[0105] (2) booster layer
[0106] Polyoxyethylene 65g
[0108] Povidone 5g
[0109] Iron oxide red 1g
[0110] Magnesium Stearate 1g
[0111] 75% ethanol appropriate amount
[0112] Makes 1000 pieces
[0113] (3) Prescription of coating solution (1000 tablets dosage)
[0114] Cellulose acetate 20g
[0115] Polyethylene glycol 1g
[0116] Triethyl citrate 2g
[0117] Proper amount of ethanol
[0118] Preparation:
[0119] 1. Sieve and mix the raw and auxiliary materials of the drug-containing layer and the booster layer according to the above-mentioned prescription amount, respectively, and mix wet granulation, and use the two-time compression technology to compress the drug-containing layer and the booster layer into a double-lay...
Embodiment 3
[0122] (1) Drug-containing layer
[0123] Felodipine 10g
[0125] Polyoxyethylene 130g
[0126] Hypromellose 9g
[0127] Magnesium Stearate 1g
[0128] (2) booster layer
[0129] Polyoxyethylene 70g
[0130] Sodium chloride 30g
[0131] Hypromellose 5.5g
[0132] Iron oxide red 1g
[0133] Magnesium stearate 0.5g
[0134] Makes 1000 pieces
[0135] (3) Prescription of coating solution (1000 tablets dosage)
[0136] Cellulose acetate 60g
[0137] Polyethylene glycol 3g
[0138] Proper amount of ethanol
[0139] Proper amount of acetone
[0140] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com