Platelet aggregation resistant chimeric monoclonal antibody and/or its fragment
An anti-platelet aggregation and monoclonal antibody technology, applied in the direction of antibodies, hybrid peptides, blood diseases, etc., can solve the problems of increasing the probability of immune response, weakening, and destroying the therapeutic effect, so as to reduce cardiac ischemic events and prevent acute The effect of thrombus formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
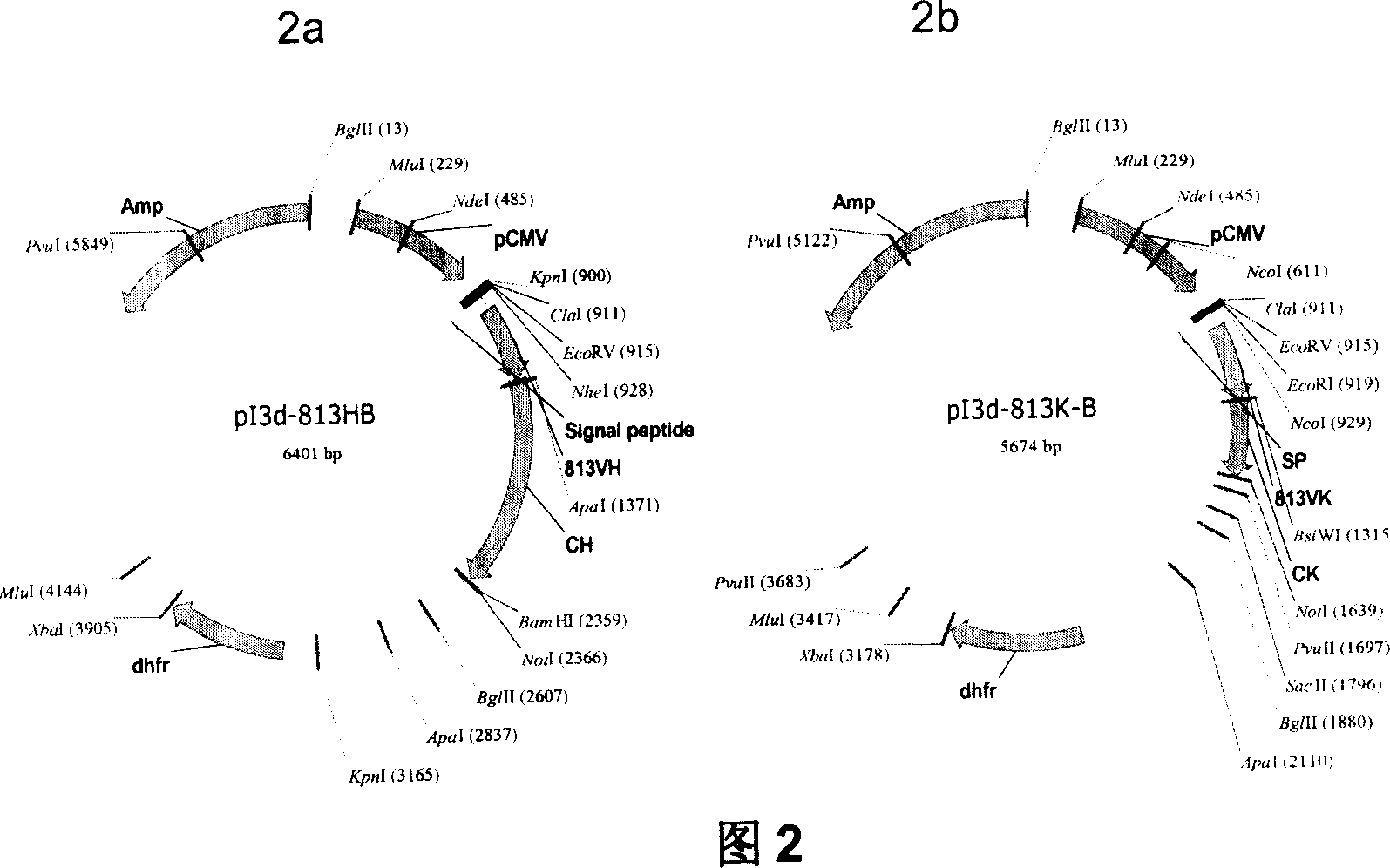
[0041] Example 1 Construction of Chimeric 813 Antibody Eukaryotic Expression Plasmid
[0042] 1. Construction of heavy chain and light chain variable region cDNA of monoclonal antibody
[0043] Cloning of the variable region sequence of the anti-human platelet glycoprotein IIIa monoclonal antibody hybridoma cell line (R813), using 5'RACE (Rapid Amplification of cDNA Ends, rapid amplification of cDNA ends) technology, from secreted anti-human platelet The variable region sequence of the functional antibody cloned in hybridoma cell line 813 of glycoprotein IIIa murine monoclonal antibody. The steps can be briefly described as follows: an antisense gene-specific primer (GSP1) is used to synthesize the first strand of cDNA, after the first strand of cDNA is purified, terminal deoxynucleotidy transferase (Terminal deoxynucleotidy transferase, TdT) is used to generate A synthetic homopolynucleotide anchor sequence is added to the 3′ end of the DNA. The cDNA is amplified using a se...
Embodiment 2
[0106] Example 2 Expression and Screening of Chimeric 813 Antibody
[0107] Material:
[0108] -dhfr-deficient CHO cells were purchased from Shanghai Cell Institute, Chinese Academy of Sciences
[0109] - Calcium transfection kit (Invitrogen)
[0110] -MTX (Sigma Corporation)
[0111] 1. Cell line transfection, using the calcium phosphate method for transfection.
[0112] Plasmid DNA was linearized with PvuI digestion. The transfection method was strictly operated according to the method of the kit. Cultivate the cells under standard growth conditions for 24 hours, take the supernatant for ELISA to detect the transient expression level; after confirming that the transfected cells can secrete and express antibodies, digest the cells, and use selective medium (excluding H and T) at 1000-8000 Ratio of cells / well Divide the cells into 96-well plates at 37°C, 5% CO 2 down to cultivate.
[0113] 2. Screening of high expression cell lines
[0114] (1) After monoclonal cells a...
Embodiment 3
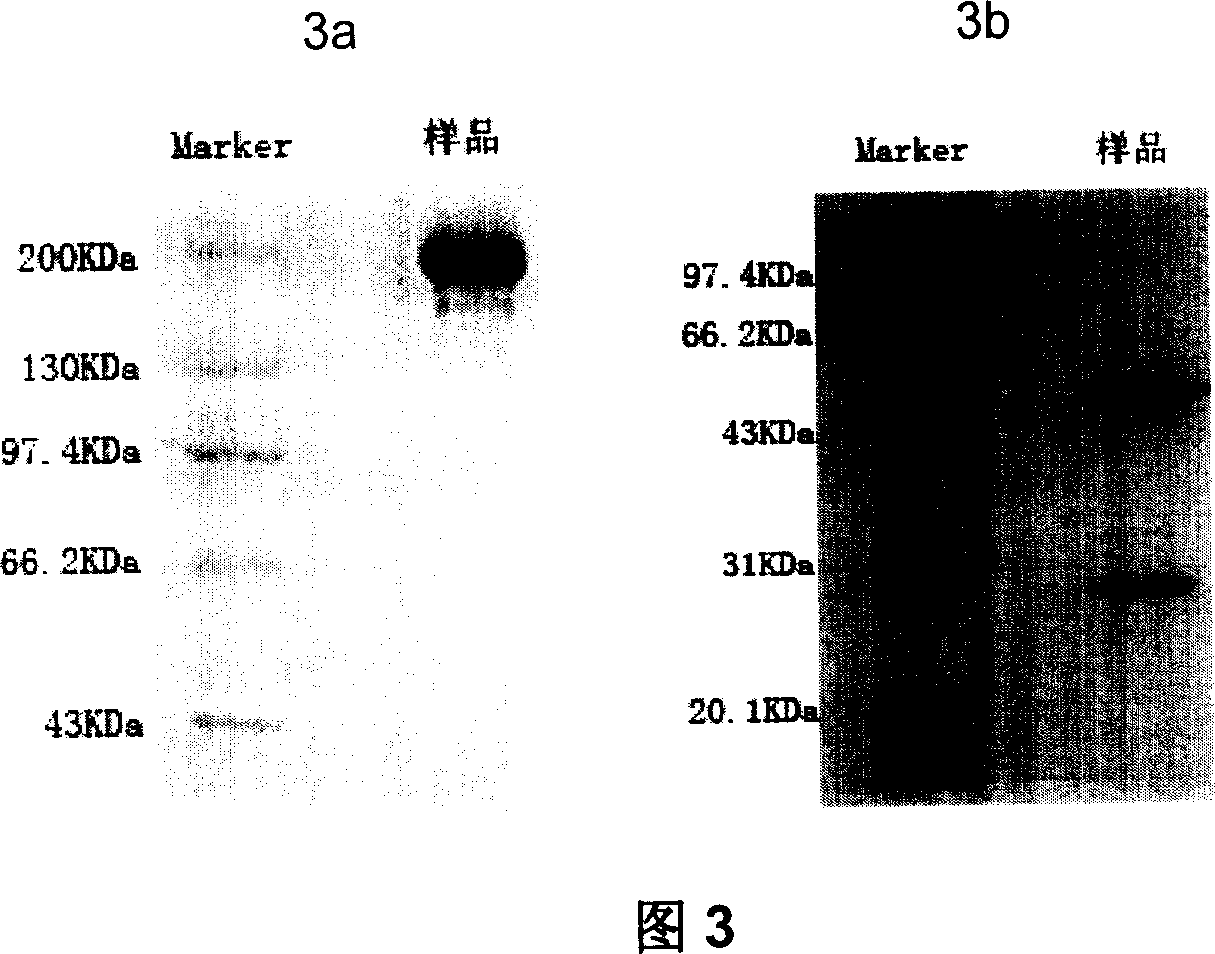
[0120] Example 3 Chimeric 813 Antibody and Chimeric 813 Antibody F(ab') 2 Fragment purification
[0121] 1. Purification of chimeric 813 antibody
[0122] Chimeric antibodies were purified by protein A affinity chromatography. The specific steps are: (1) washing the protein A affinity chromatography column with 3-5 times column volume of water. (2) Equilibrate protein A affinity chromatography column with 20mM phosphate buffer, pH 7.0. (3) Pump the cell culture supernatant containing the desired purified monoclonal antibody into the protein A affinity chromatography column. (4) Wash the column with 20mM phosphate buffer, pH 7.0, to OD 280 <0.01. (5) Elute with 0.1M citric acid-sodium citrate buffer, pH 3.0, collect the eluted peak, and adjust the pH value of the collected antibody solution to be neutral with 1M Tris-HCl buffer, pH 9.0. (6) Dialysis desalination. After purification, the chimeric 813 antibody was determined by HPLC, and its purity was 94.4%. The electrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com