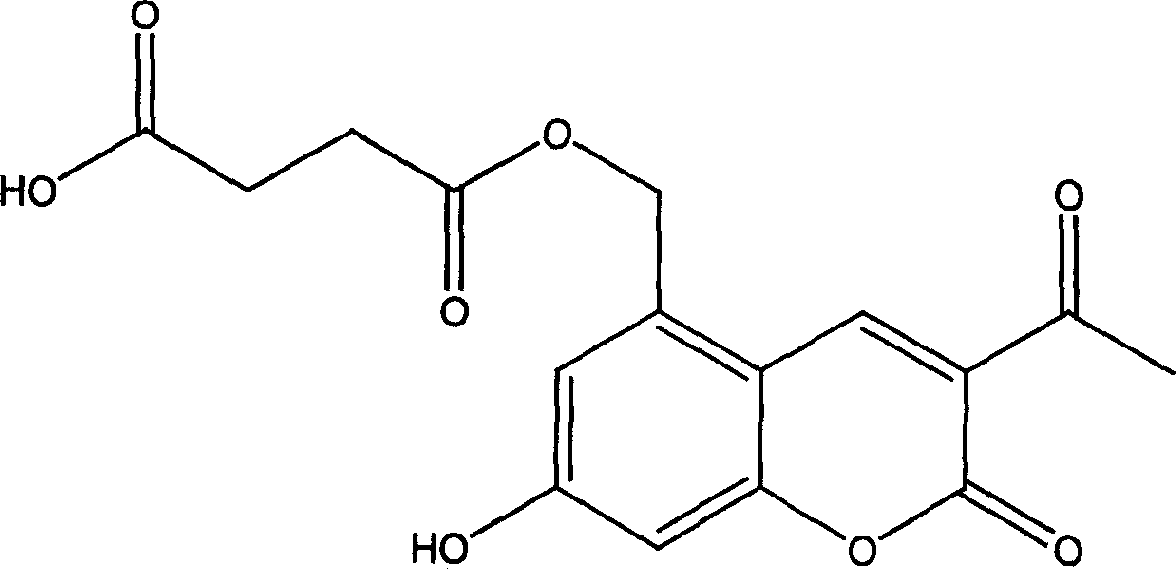
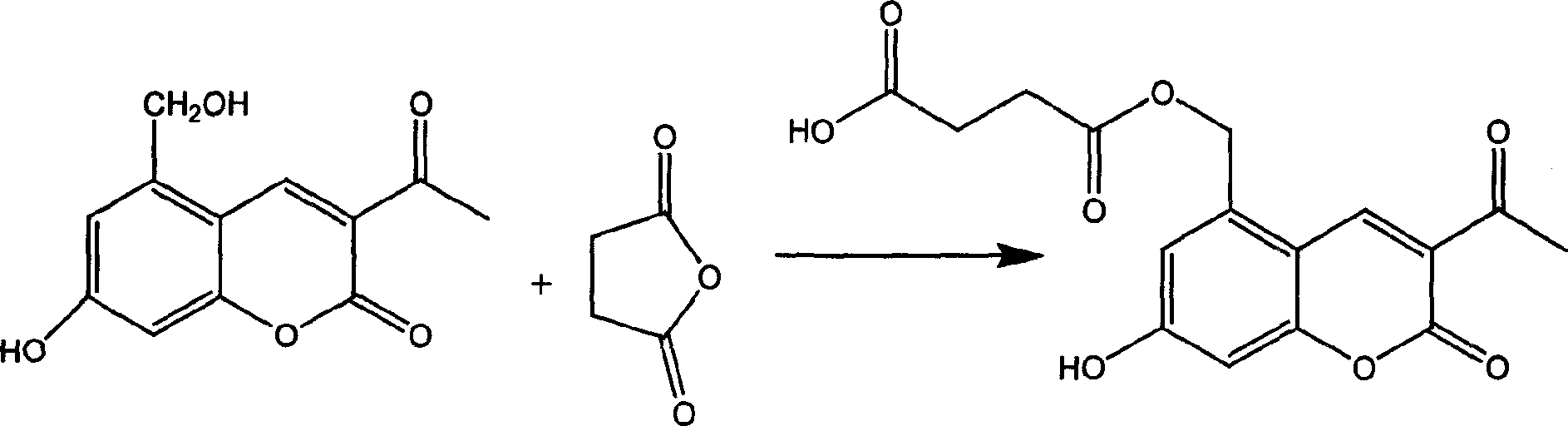
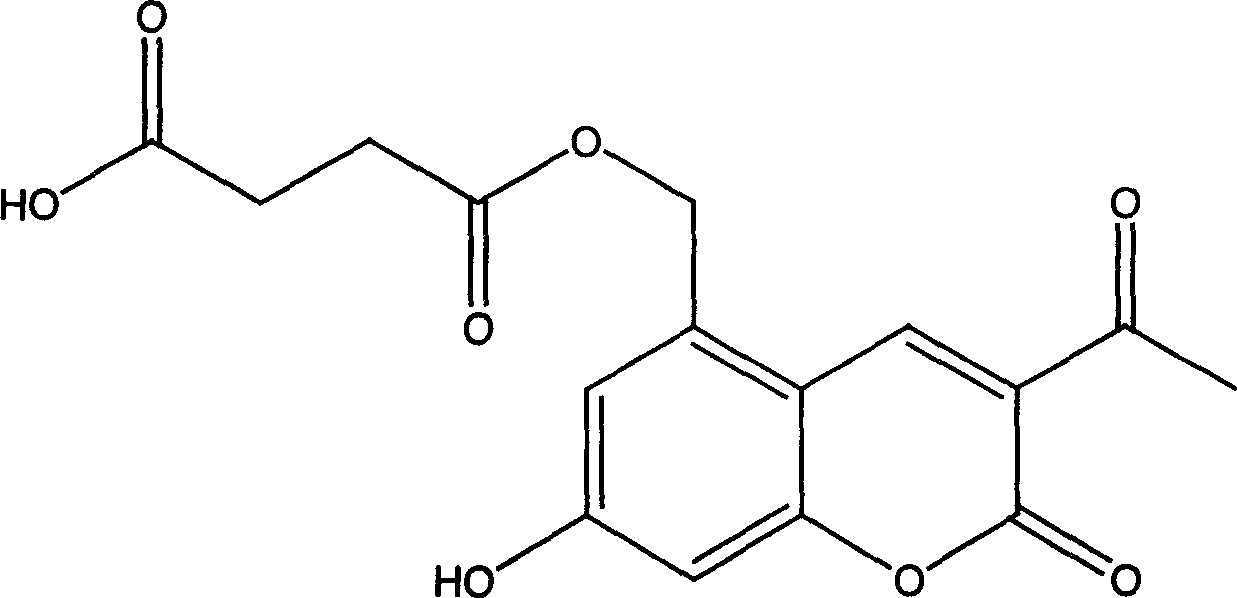
Armiharisin succinic mono ester with cholagogic effect, and its salt and medicinal composition
A technology of succinic acid monoester and leucicillin A, applied in the field of medicine, can solve problems such as toxic side effects and lower drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Preparation of Leucocin A Succinate Monoester (Method 1)
[0018] Add 20g (0.085mol) of Leucocin A, 46.9g (0.34 mol) of anhydrous potassium carbonate, 300ml of ethyl acetate and 100ml of anhydrous N,N-dimethylformamide into a 500ml round bottom flask, oil bath 109 Reflux for 1 hour at °C. A yellow solid gradually precipitated from the reaction liquid. 34g (0.34mol) of succinic anhydride was added, the solid part of the reaction solution was dissolved, and the reaction was refluxed for 30 hours, cooled to room temperature, and filtered with suction. The obtained solid was washed with a small amount of ethyl acetate. The filter cake was dissolved in an appropriate amount of water, and 10% hydrochloric acid was used. Adjust to pH 3.5, stir for 0.5 hours, and filter with suction to obtain part of the product. Concentrate the filtrate to recover ethyl acetate, add 200 ml of water to the concentrate, adjust the pH to 3.5 with 10% hydrochloric acid, stir for 0.5 hours, a...
Embodiment 2
[0089] Example 2 Preparation of Leucocin A Succinate Sodium
[0090] Dissolve 1.67g (5mmol) of Leucocin A succinate monoester in 60ml of methanol, add 0.42g (5mmol) of sodium bicarbonate powder at room temperature while stirring, and after stirring at room temperature for 20 minutes, a large amount of yellow-green solid precipitates out, continue to stir and react 3 After hours, suction filtration, the filter cake was washed with a small amount of methanol, and after vacuum drying, 1.44 g of yellow-green powdery solid was obtained, mp197-198.5°C, and the yield was 81%.
Embodiment 3
[0091] Example 3 Preparation of leucine A succinate monoester lysine salt
[0092] Dissolve 1.67g (5mmol) of Leucocin A succinate monoester in 30ml of tetrahydrofuran, add 0.82g (5mmol) of lysine at room temperature while stirring, and react with stirring for 12 hours at room temperature. Quickly filter with suction to obtain a light yellow solid. Transfer to a vacuum dryer, vacuum drying at 50°C for 4 hours to obtain product 2.2g, mp175-177°C, yield 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com