Lithium-lanthanum-silicon-sulfur solid electrolyte material for secondary lithium cell and its preparing method
A solid electrolyte and secondary lithium battery technology, which is applied in secondary battery manufacturing, secondary batteries, secondary battery components, etc., can solve the problems of conductive cation migration and binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
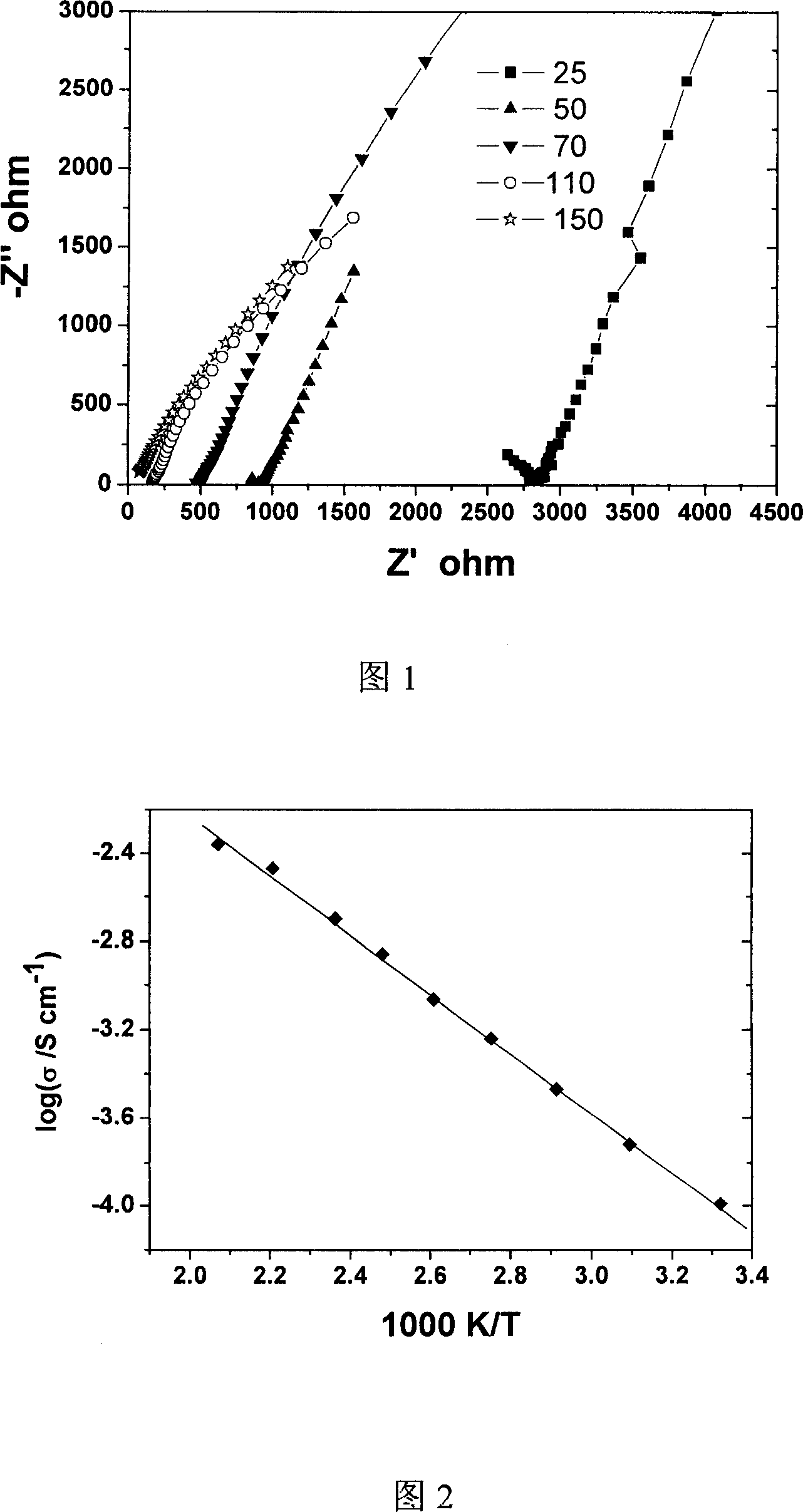
[0027] Using Li 2 S and SiS 2 and La 2 S 3 Powder (purity respectively 99%, 99.0%, 99.0%)) was weighed according to the molar ratio of 6:0.5:3 and loaded into a high-energy ball mill jar (ZrO 2 material), high-energy ball milling under the protection of argon atmosphere for 10 hours, and finally pressed into tablets (diameter 10mm, thickness 1mm, pressure 8MPa), a layer of indium film was pressed on the surface as a conductive electrode, and finally a block test of a solid electrolyte was made Material.
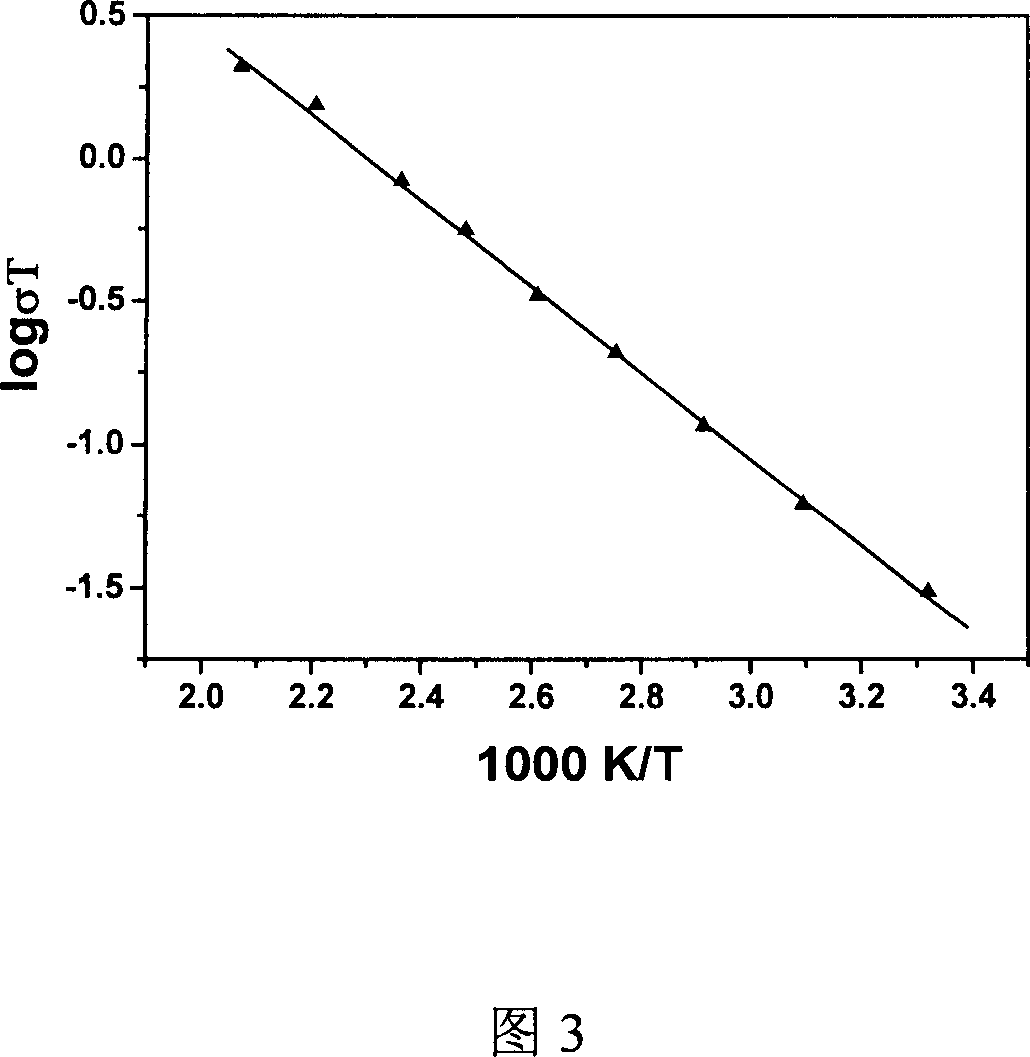
[0028] The conductivity test shows that it has ionic conductivity, and the characteristic straight line of ionic conductivity can be clearly observed at the low frequency end of the AC impedance spectrum at different temperatures. By calculation, the total conductivity at room temperature can be obtained as 5.35×10 -5 S / cm, at the same time, by plotting lgσT against 1000 / T, the ion transport activation energy of the material is (0.129eV).
Embodiment 2
[0030] Using Li 2 S, SiS 2 and La 2 S 3 Powder (purity is 99%, 99.0%, 99.0%) is weighed according to the molar ratio of 6:0.5:3 and put into glass tube, vacuumize (less than 10 -2 Pa) and seal it with a hydrogen-oxygen flame. Slowly raise the temperature of the glass tube containing the mixture to 450°C and keep it warm for 24h, then raise the temperature to 650°C-750°C for solid phase reaction, and the reaction time is 10-12h. After water-cooling and quenching, the tube was opened, and the powder was ground under the protection of an argon atmosphere. The tableting process was the same as that of Test Embodiment 1.
[0031] The performance test results are basically the same as 1, and its room temperature ionic conductivity is 1.25×10 -5 S / cm, lower than Example 1.
Embodiment 3
[0033] Using La powder, Si powder, S powder and Li 2 S powder (purity respectively 99.99%, 99.9%, 99% and 99.0%) according to 6Li 2 S-0.5La 2 S 3 -3SiS 2 The stoichiometric ratio is weighed and packed into a glass tube, and finally made into a solid electrolyte material according to the method of Embodiment 2.
[0034] The performance test results are basically the same as 1, and its room temperature conductance is about 1.0×10 -5 S / cm, lower than Examples 1 and 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com