Method for preparing double soluble noble metal nano particle
A nanoparticle and noble metal technology, which is applied in the field of preparation of double-soluble noble metal nanoparticles, can solve the problems of insolubility and other problems, and achieve the effects of rapid preparation, improved stability, and convenient biological application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
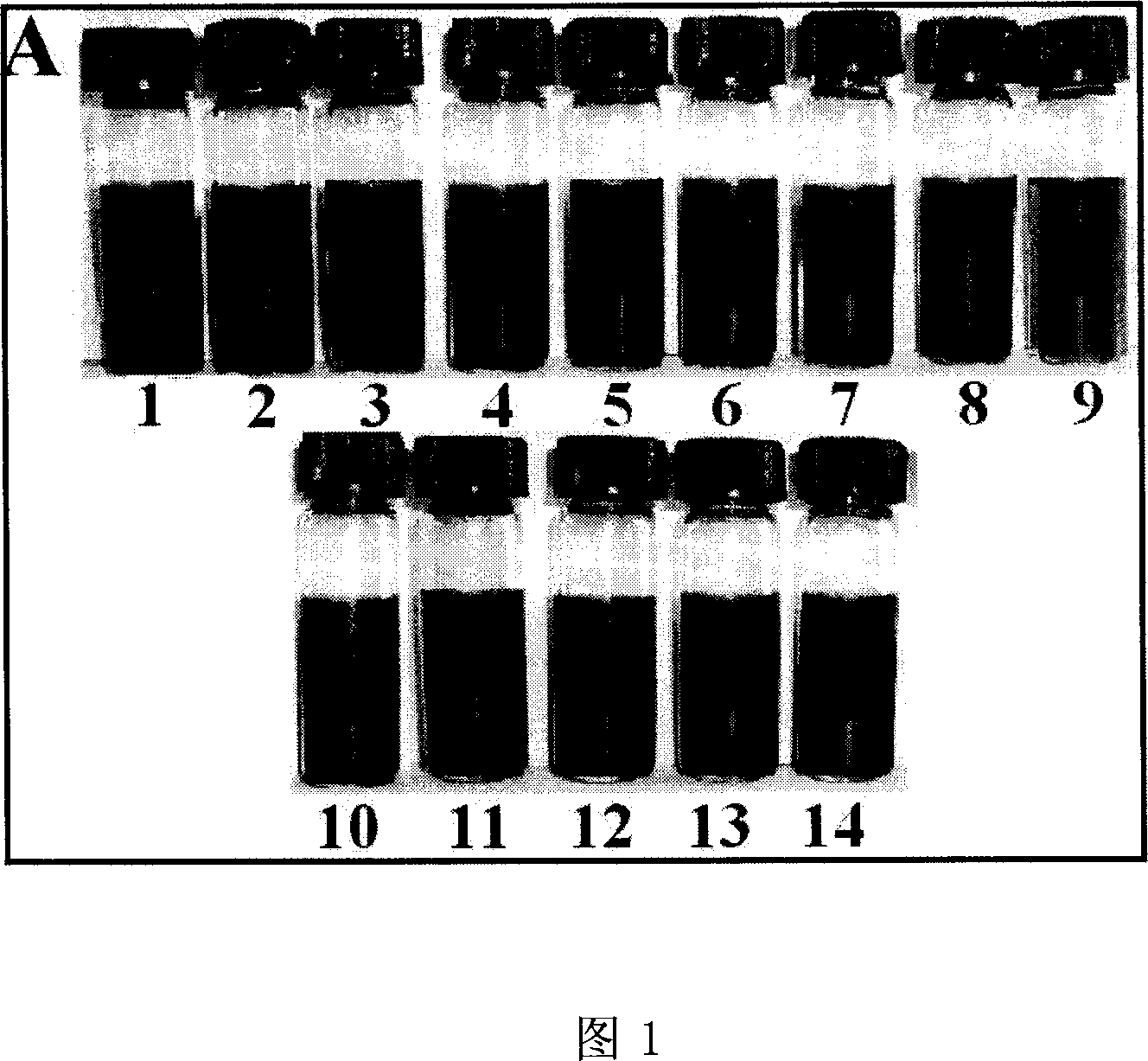
[0054] Preparation method of n-octadecylamine-protected gold nanoparticles. Draw 5.0mL of chloroauric acid (0.024M) solution with a pipette and place it in a beaker, heat and evaporate to dryness, add 0.054g of n-octadecylamine, then add 75mL of toluene to make the molar ratio of n-octadecylamine and chloroauric acid The ratio is 50:1, and the mixture is ultrasonically homogeneous. The obtained system was an orange-yellow clear solution, and 0.054 g of sodium borohydride aqueous solution (0.5 mL) was added under magnetic stirring. The solution turned red, and after continuing to stir for 12 hours, a purple gold sol was obtained.
[0055] The obtained gold sol was distilled to near dryness under reduced pressure, and 80 mL of absolute ethanol was added. The gold nanoparticles protected by n-octadecylamine precipitated after one night. n-octadecylamine and other impurities, and finally a black solid was obtained. After the sample was dried overnight in an oven at 50° C., the d...
Embodiment 2
[0057] Preparation of decylamine-protected gold nanoparticles. Draw 5.0mL of chloroauric acid (0.024M) solution with a pipette and place it in a beaker, heat and evaporate to dryness, add 0.024g of decylamine, then add 75mL of toluene, so that the molar ratio of n-decylamine to chloroauric acid is 50 : 1, mix and sonicate evenly. The obtained system was an orange-yellow clear solution, and 0.054 g of sodium borohydride aqueous solution (0.05 mL) was added under magnetic stirring. The solution turned red, and after continuing to stir for 12 hours, a red gold sol was obtained.
[0058] The obtained gold sol was distilled to near dryness under reduced pressure, and 80 mL of absolute ethanol was added, and the gold nanoparticles protected by n-decylamine precipitated after one night. Amines and other impurities were removed, and finally a black solid was obtained. After drying the sample in an oven at 50° C. overnight, the dried decylamine-protected gold nanoparticles were obtai...
Embodiment 3
[0060] Use a pipette to draw 5.0mL (0.024M) of silver nitrate solution into a beaker, heat and evaporate to dryness, add 0.054g of n-octadecylamine, then add 75mL of toluene to make the molar ratio of n-octadecylamine to chloroauric acid 50:1, mixed ultrasonically evenly. The obtained system was an orange-yellow clear solution, and 0.054 g of sodium borohydride aqueous solution was added under magnetic stirring. After continuing to stir for 12 hours, a yellow silver sol was obtained.
[0061] The obtained silver sol was distilled to nearly dryness under reduced pressure, and 80 mL of absolute ethanol was added, and the silver nanoparticles protected by n-octadecylamine precipitated after one night, and after the precipitate was separated by centrifugation, absolute ethanol was added and ultrasonically dispersed, and the free n-octadecylamine and other impurities, and finally a black solid was obtained. After the sample was dried overnight in an oven at 50° C., the dried silve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com