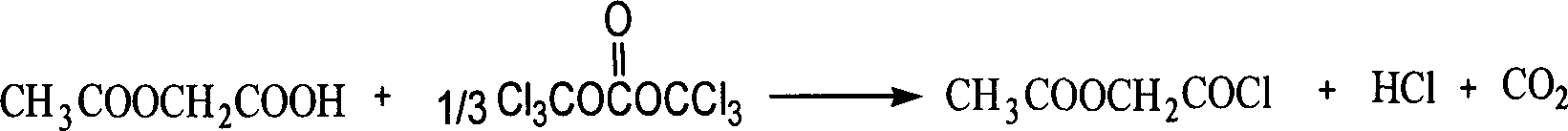Acetoxy acetyl chloride chemical synthesizing method
A technology of acetoxyacetyl and acetoxyacetic acid, applied in the field of chemical synthesis of acetoxyacetyl chloride, can solve the problems of high sealing requirements of reaction equipment, low product yield and purity, and high production cost, and achieves a large implementation Value and social and economic benefits, low production cost, and low three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The amount ratio of feed material is acetoxyacetic acid: two (trichloromethyl) carbonate: organic amine catalyst is 1: 0.34: 0.01, and catalyzer is tetramethylguanidine, and organic solvent is toluene, and its consumption is the quality of acetoxyacetic acid 2 times.
[0018] In a 100mL three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 11.8g (100mmol) of acetoxyacetic acid, 10.1g (34mmol) of bis(trichloromethyl)carbonate, 28ml of toluene and tetramethylguanidine 0.1 g (1 mmol). After the addition was completed, the temperature was raised to 40°C and reacted at 40°C for 7 hours. After the reaction was completed, the solvent was evaporated under normal pressure, and the vacuum degree was 1.6kpa. 90.3%, purity 98.5%. 1 H NMR (CDCl 3 )δ: 4.9(s, 2H), 2.2(s, 3H).
Embodiment 2
[0020] The ratio of the amount of feed material to acetoxyacetic acid: bis(trichloromethyl)carbonate: organic amine catalyst is 1: 0.7: 0.01, and the feeding amount of acetoxyacetic acid is 11.8g (100mmol), and bis(trichloromethyl) Carbonate charging capacity is 20.5g (70mmol), and catalyzer is 1,3-dimethyl-2-imidazolidinone, and its consumption is 0.1g (1mmol), and organic solvent is toluene, and its consumption is the quality of acetoxyacetic acid 2 times.
[0021] The reaction temperature was 45-50° C., and other operations were the same as in Example 1 to obtain 12.5 g of acetoxyacetyl chloride with a product yield of 91.5% and a purity of 99.0%. 1 H NMR (CDCl 3 )δ: 4.9(s, 2H), 2.2(s, 3H).
Embodiment 3
[0023] The ratio of the amount of feed material to acetoxyacetic acid: bis (trichloromethyl) carbonate: organic amine catalyst is 1: 1: 0.02, and the feeding amount of acetoxyacetic acid is 11.8g (100mmol), bis (trichloromethyl) The charging amount of carbonate is 29.7g (100mmol), the catalyst is pyridine, and its consumption is 0.2g (2mmol), and the organic solvent is tetrahydrofuran, and its consumption is 2 times of the mass of acetoxyacetic acid.
[0024] The reaction temperature was 60-65° C., and other operations were the same as in Example 1 to obtain 11.8 g of acetoxyacetyl chloride with a product yield of 86.6% and a purity of 99.0%. 1 H NMR (CDCl 3 )δ: 4.9(s, 2H), 2.2(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com