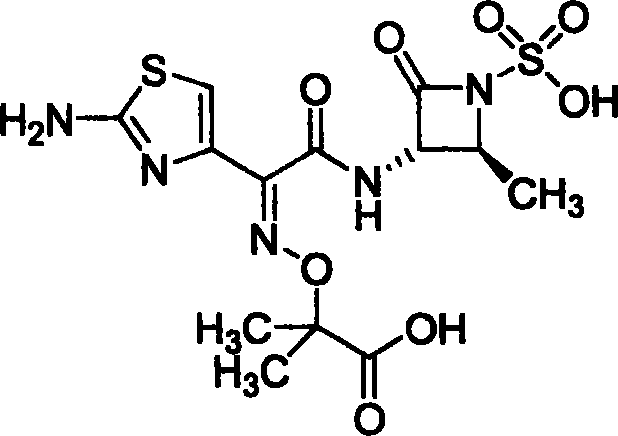
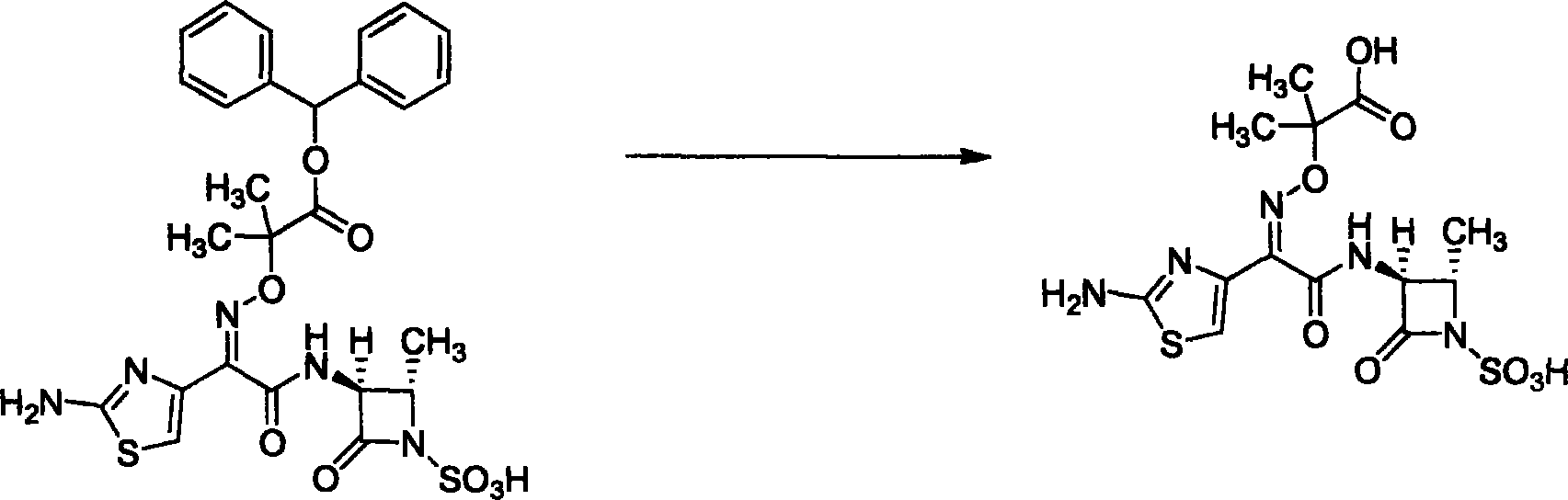
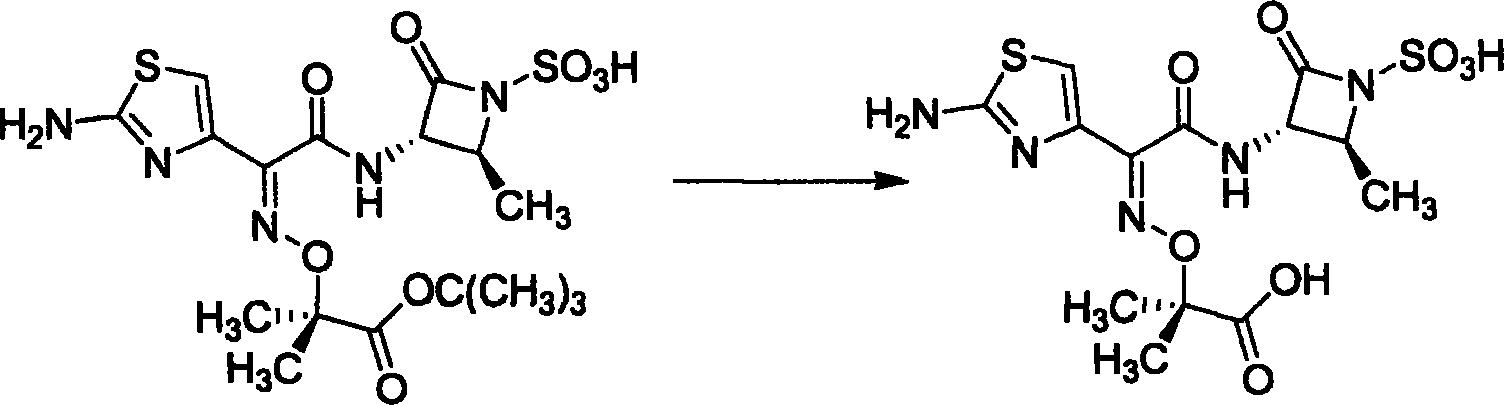Prepn process of aztreonam
A technology of aztreonam and benzhydryl aztreonam, applied in the direction of organic chemistry, can solve the problems of high cost of trifluoroacetic acid, inability to recycle and apply, and inability to recycle, and achieve low cost, high yield, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of aztreonam by deprotection of tert-butyl aztreonam
[0024] Add 20g of tert-butylaztreonam and 100ml of anhydrous formic acid into a 250ml flask, stir and react at room temperature for 3 hours, after the reaction is completed, recover formic acid under reduced pressure, add 100ml of deionized water to the obtained oil, and stir at 0°C to 5°C After half an hour, a white solid was formed, filtered, the filter cake was washed with cold water and cold acetone, and dried to obtain 13.3 g of the product. Yield 75%.
Embodiment 2
[0025] Embodiment 2: Preparation of aztreonam by deprotection of tert-butyl aztreonam
[0026] Add 20g of tert-butylaztreonam and 100ml of 50% formic acid aqueous solution into a 250ml flask, stir and react at room temperature for 3 hours, after the reaction is complete, cool to 0°C-5°C and stir for 1 hour, a white solid is formed, filter, and collect the obtained formic acid aqueous solution Apply mechanically, filter cake is washed with cold water and cold acetone, and drying obtains 11.3g aztreonam product. Yield 63.8%.
Embodiment 3
[0027] Embodiment 3: benzhydryl aztreonam deprotection preparation aztreonam
[0028] Add 20g of benzhydryl aztreonam and 100ml of anhydrous formic acid into a 250ml flask, stir and react at room temperature for 3 hours, after the reaction is completed, recover formic acid under reduced pressure, add 100ml of deionized water to the obtained oil, After stirring for half an hour, a white solid was formed, filtered, the filter cake was washed with cold water and cold acetone, and dried to obtain 9.8 g of the product. Yield 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com