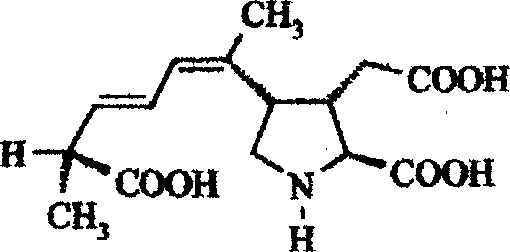
Amnestic shellfish-poison competitive immue quantitative detection reagent box, its preparation and use
An enzyme-linked immunosorbent immunoassay and quantitative detection technology, which is applied in the field of amnestic shellfish poison competitive enzyme-linked immunosorbent immunoassay kits, can solve the problems of complex matrix and achieve high repeatability, good specificity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of sample antigen: remove the shell, wash the shellfish with double distilled water, and homogenize with a homogenizer; add 70mL of 50% methanol solution to 10.0g sample, and stir for 5min; centrifuge at 3000g for 10min; take 100μL The supernatant was diluted 1:10 with double distilled water (1+9); take 50 μL for analysis with the kit, and the dilution factor at this time is 80;
Embodiment 2
[0028] Example 2: Preparation of monoclonal antibody and detection of monoclonal antibody properties
[0029] After dissolving 10 grams of ASP (DA) samples in 300 milliliters of pyridine according to the mixed anhydride method, add 50 milliliters of succinic anhydride, stir until the reaction is complete, wash with acid water, filter the precipitate with suction, and dry to obtain a crude product. The crude product was dissolved in acetone, mixed with an appropriate amount of silica gel, dried in the air, separated by column chromatography on a silica gel column, monitored by TLC, and the points of Rf=0.09 (developing solvent, petroleum ether:acetone=3:1) were collected and combined, Dry at room temperature to obtain activated chondroitin (DA).
[0030] Dissolve 50 mg of the above substance in 5 mL of dioxane, place at 4°C for 10 min, add 25 µL of tri-n-butylamine, mix well, then add 19 µL of isobutyl chloroformate, stir magnetically at 4°C for 30 min, and this is liquid A. A...
Embodiment 3
[0037] Example 3: Determination of Molecular Weight of Monoclonal Antibody
[0038] In the Bio-Rad electrophoresis tank, pour 12% separation gel, and add a small amount of water, so that the edge of the gel is horizontal after solidification. The filter paper was sucked to remove water, poured into the electrode buffer, and electrophoresed at a constant current (I=12mA) for 10min. After aspirating the electrode buffer, pour 3% stacking gel into the tank. After gelation, 10 μl of purified monoclonal antibody and standard protein samples were added to each sample well. The sample was concentrated in the stacking gel for 20min at a constant flow (I=20mA), and then separated in the separating gel for 2.5h. After the separation, the separating gel was fixed, dyed, decolorized and dried (the second edition of Molecular Depression Test Guidelines, 1999). In SDS-PAGE discontinuous electrophoresis, the relative mobility Mr of each protein (the ratio of the distance the protein migra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com