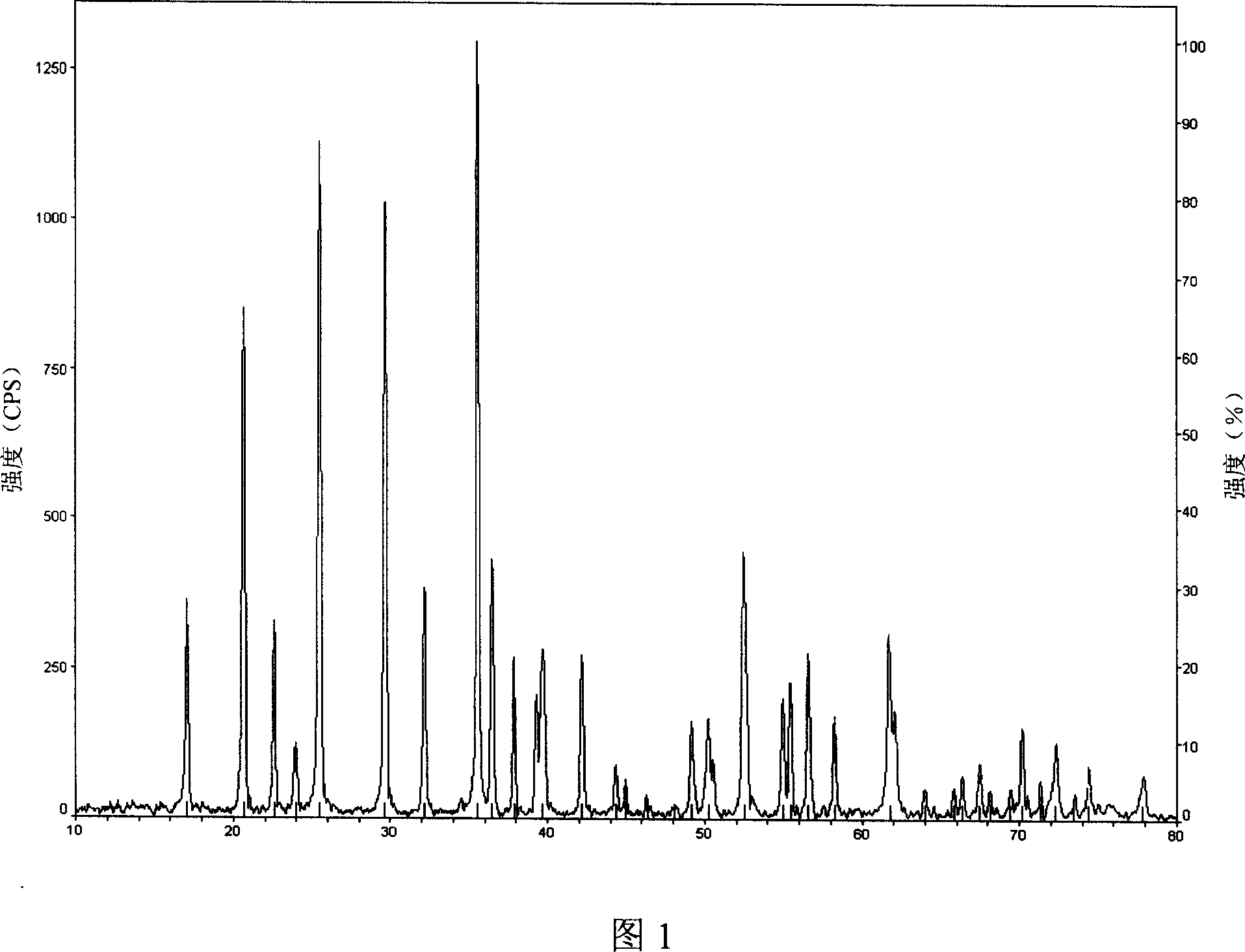
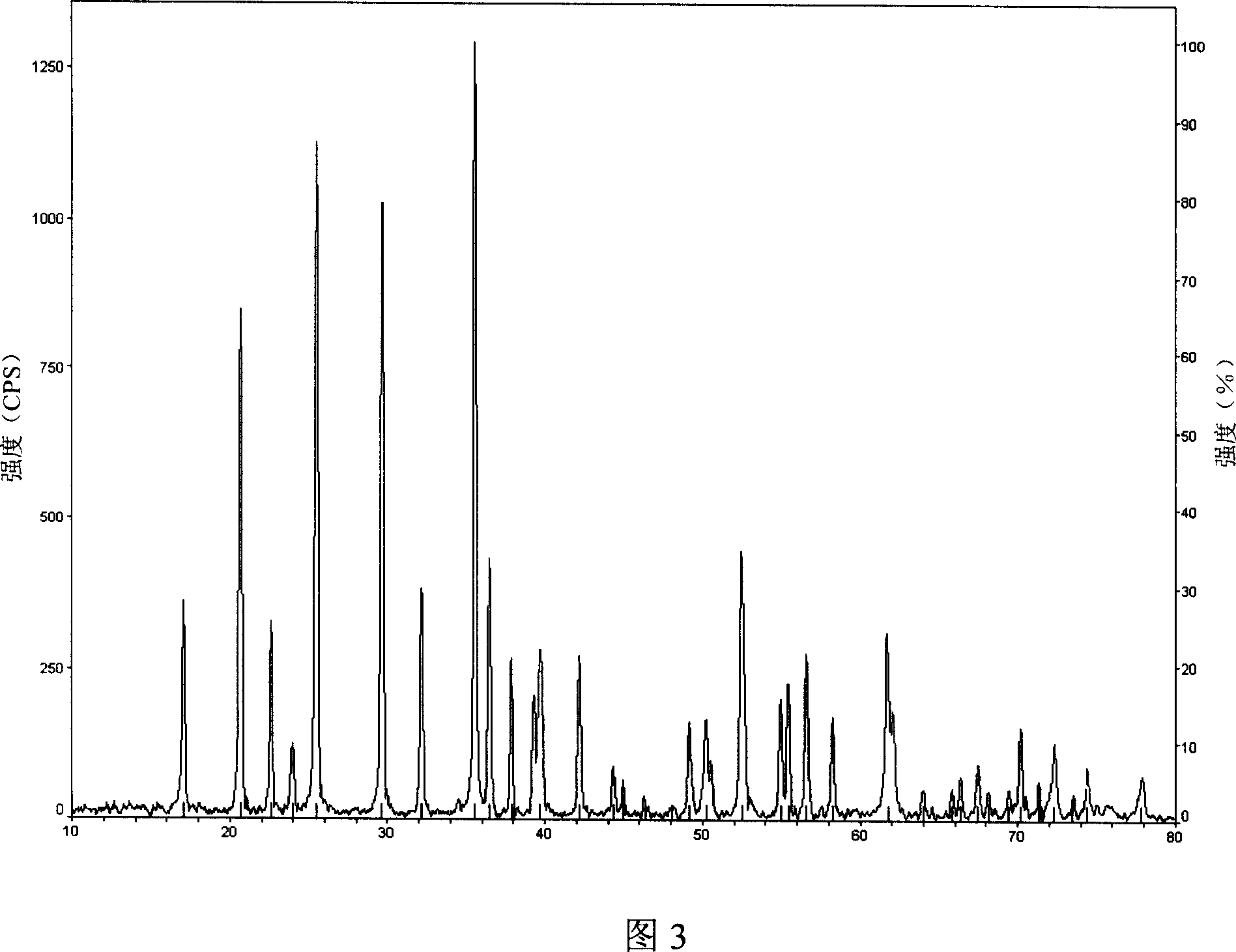
Method for producing active substance ferrous lithium phosphate as lithium-ion battery anode
A cathode active material, lithium iron phosphate technology, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problems of low specific capacity, high cost, poor cycle performance, etc., to achieve high specific capacity, low cost, The effect of excellent electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This example is used to illustrate the preparation method of lithium ferrous phosphate provided by the present invention.
[0044] Dissolve 20 g of polyvinyl alcohol in 200 ml of deionized water under electromagnetic stirring, and then add 0.05 mol of lithium hydroxide monohydrate and 0.05 mol of NH 4 h 2 PO 4 , stir and mix evenly and then add 0.025 mole of Fe 2 o 3 The powder was stirred for 2 hours and then heated to 90°C to evaporate the water. During this process, it was stirred and granulated. After 2 hours, it was placed in an oven at 120°C for 12 hours to obtain a gel. The obtained gel was placed in an alumina crucible, placed in a high-temperature resistance furnace, and sintered at a rate of 10°C / min to 120°C under a mixed atmosphere of nitrogen / hydrogen (partial pressure of hydrogen was 8%), at a constant temperature of 1 After one hour, raise the temperature at 6°C / min to 300°C for one-stage sintering, and after constant temperature for 4 hours, then inc...
Embodiment 2
[0046] This example is used to illustrate the preparation method of lithium ferrous phosphate provided by the present invention.
[0047] Dissolve 20 g of polyacrylamide in 200 ml of deionized water under electromagnetic stirring, and then add 0.102 mol of LiNO 3 and 0.1 mol NH 4 h 2 PO 4 , stir and mix well, then add 0.05 mole of Fe 2 CO 3 The powder was stirred for 2 hours and then heated to 80°C to evaporate the water. During this process, it was stirred and granulated. After 4 hours, it was put into an oven at 200°C to solidify for 2 hours to obtain a gel. Place the obtained gel in an alumina crucible, put it into a high-temperature resistance furnace, and carry out one-stage sintering at a rate of 2°C / min to 300°C under a mixed atmosphere of argon / hydrogen (hydrogen partial pressure is 5%), and constant temperature After 4 hours, the temperature was raised to 650°C at 10°C / min for two-stage sintering, and the temperature was kept constant for 10 hours, then cooled wi...
Embodiment 3
[0049] This example is used to illustrate the preparation method of lithium ferrous phosphate provided by the present invention.
[0050] Dissolve 30 g of phenolic resin in 200 ml of absolute ethanol under electromagnetic stirring, add 0.05 mol of LiCl and 0.001 mol of Mn(NO 3 ) 2 , stir well and then add 0.05 mole of FePO 4Powder, after stirring for 4 hours, heat up to 70°C to evaporate the solvent, after stirring for 2 hours, heat up to 90°C to evaporate the water, keep stirring and granulate during this process, after 2 hours, put it in an oven at 100°C for 14 hours to obtain a gel colloid. The obtained gel is placed in a crucible, placed in a high-temperature resistance furnace, raised to 350°C at 6°C / min under a nitrogen / hydrogen (partial pressure of hydrogen is 8%) mixed atmosphere, kept at a constant temperature for 4 hours, and then heated at a temperature of 10°C. ℃ / minute to 750 ℃, constant temperature for 8 hours and then cooling with the furnace to obtain a blac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com