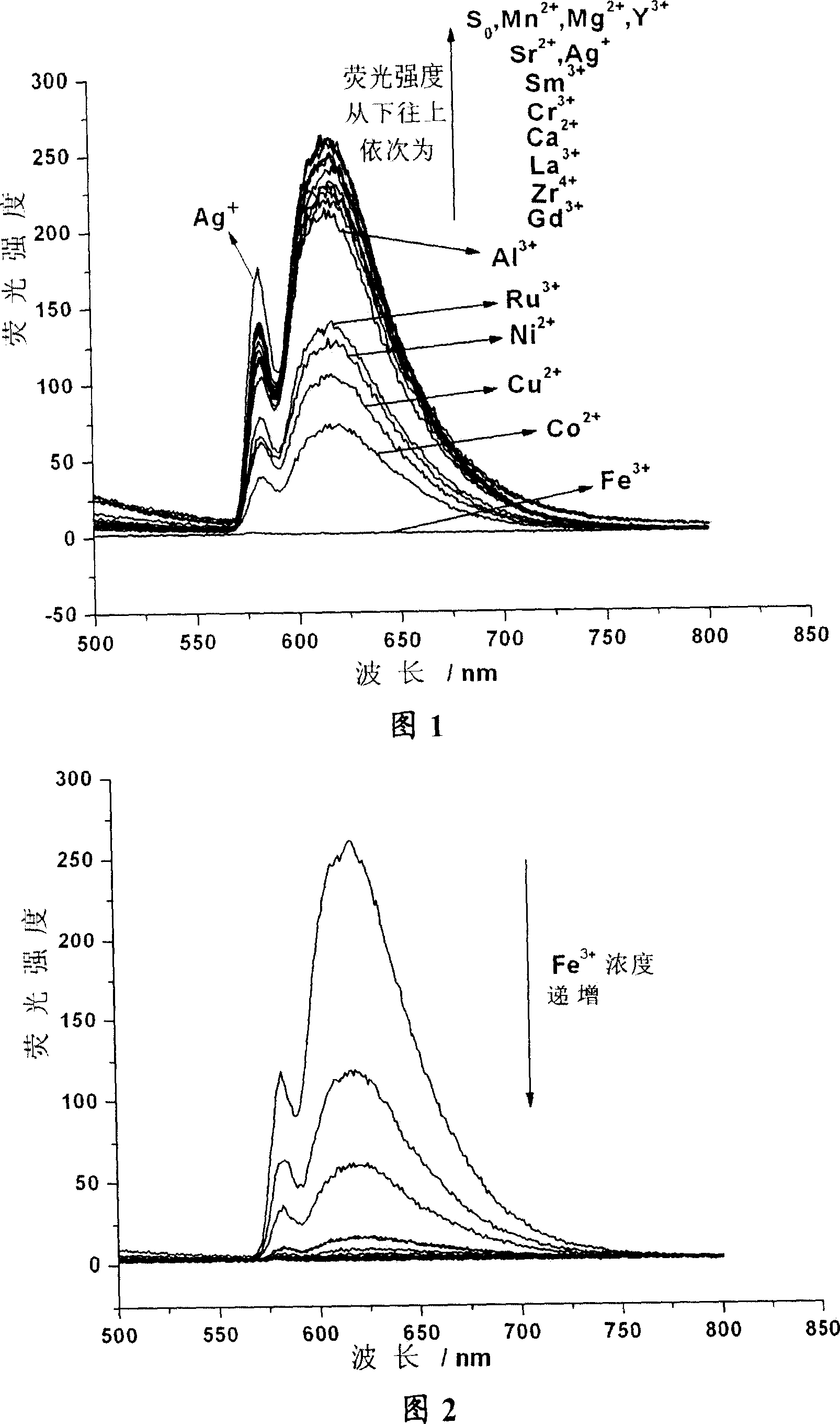
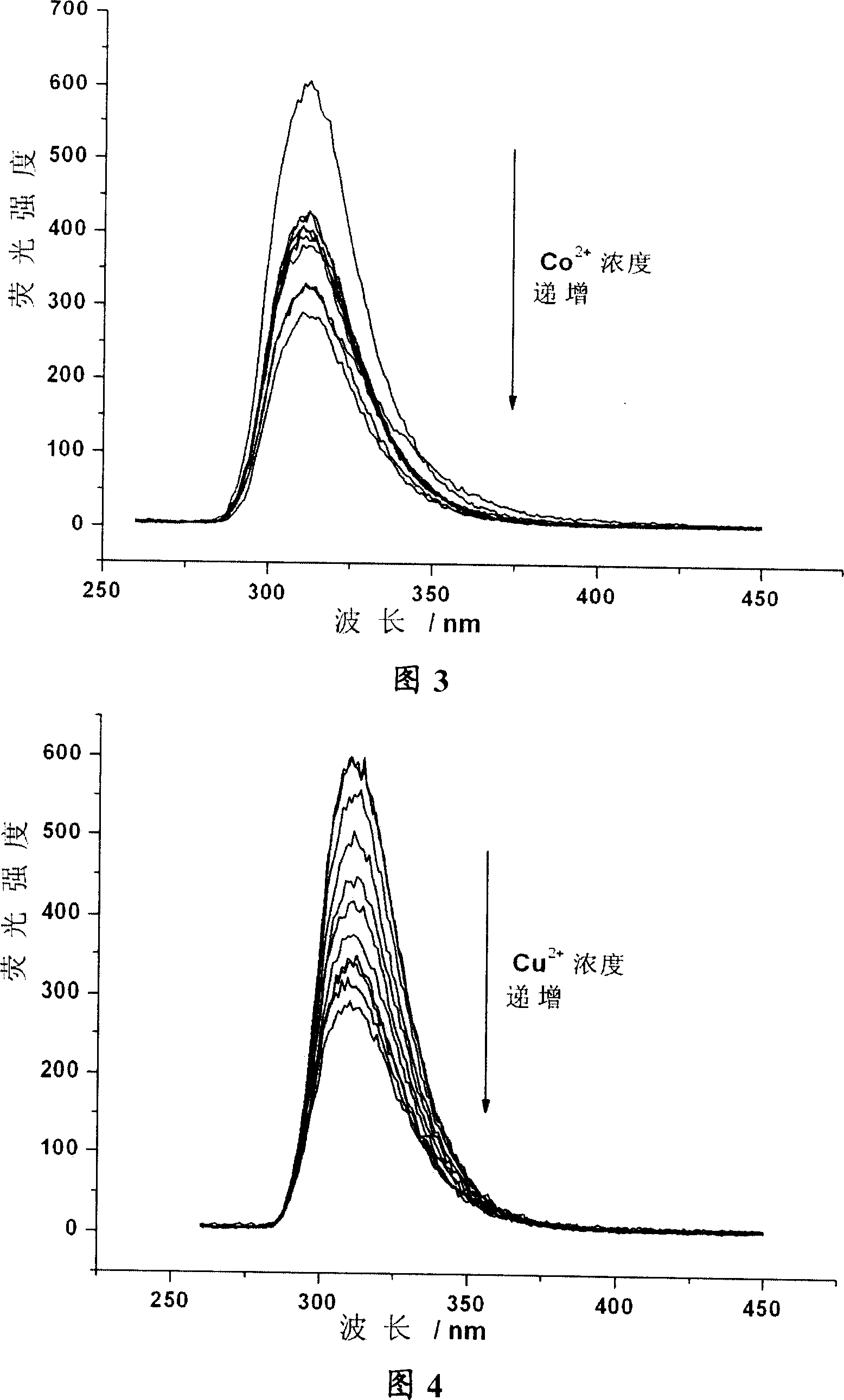
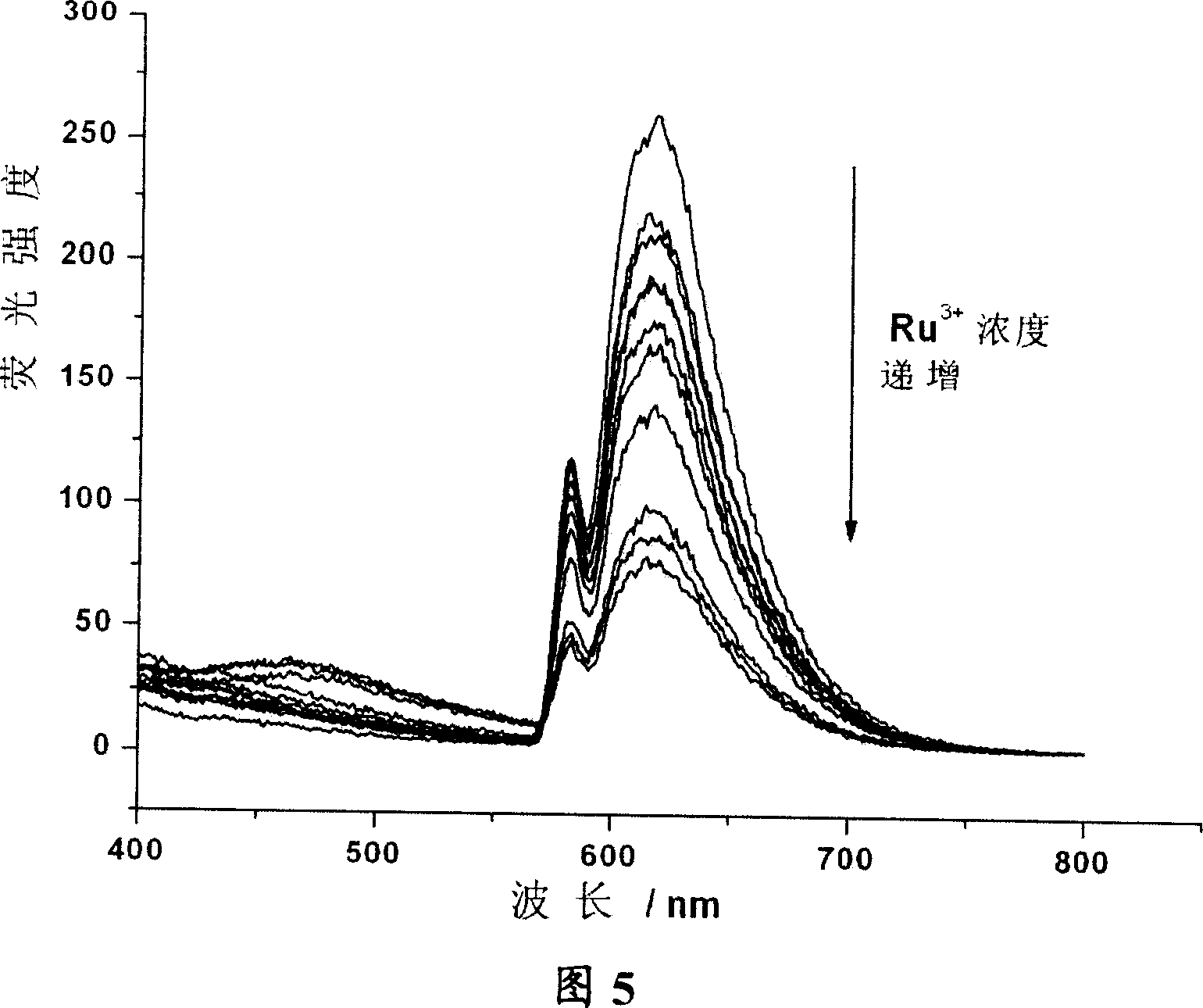
Calixarene aza-crown ether derivative, its preparation and use as metal ion fluorescent identification agent
An azacrown ether and calixarene technology, which is applied in the field of new compounds and can solve the problems of long waiting time and troublesome handling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 25,27-bis(3-bromopropoxy)-26,28-dihydroxy-5,11,17,23-tetra-tert-butylcalix[4]arene
[0028] In a container equipped with a reflux condenser, inject acetonitrile (250 ml) dried over anhydrous magnesium sulfate for 24 hours, then add p-tert-butylcalix [4] arene (6.46 g, 10 mmol), 1,3- Dibromopropane (10.2 mL, 100 mmol) and anhydrous potassium carbonate (3.45 g, 25 mmol). Heated and stirred to reflux for 48 hours, cooled, and the solvent acetonitrile was evaporated to dryness under reduced pressure. Chloroform (200 ml) was added to the obtained solid, and washed with 5% hydrochloric acid, saturated aqueous sodium bicarbonate solution, and distilled water, each washing three times, using 100 ml each time. Add 10 g of anhydrous sodium sulfate to chloroform and dry for 24 hours. The desiccant magnesium sulfate was filtered off, and the solvent chloroform was rotary evaporated under reduced pressure to obtain a white solid (slightly yellowish) crude product....
Embodiment 2
[0030] Preparation of Serine Ethyl Ester Hydrochloride
[0031]Add redistilled absolute ethanol (100 ml) into a three-necked flask equipped with a condenser, an exhaust gas absorption device, and an air inlet device. Dry hydrogen chloride was passed through for 1 hour, so that absolute ethanol was saturated with respect to hydrogen chloride. Then cool in an ice bath, add 3.5 g of serine, and continue stirring with hydrogen chloride under the ice bath for 1 hour. The ice bath was removed, and heated to reflux for half an hour. After cooling, add 10 g of anhydrous magnesium sulfate and dry for 24 hours. The desiccant was filtered off, half of the solvent was evaporated, and the product was left standing overnight in the refrigerator, and colorless crystals were precipitated, filtered, and vacuum-dried to obtain a colorless crystalline product. Melting point: 160-160.8°C, literature value 159-162°C. IR (KBr, ν, cm -1 ): 3384.3, 2975.0, 1978.6, 1746.6, 1583.1. These values...
Embodiment 3
[0033] Preparation of compounds of the present invention
[0034] In a container equipped with a reflux condenser, inject acetonitrile (200 ml) dried over anhydrous magnesium sulfate for 24 hours, and then add 25,27-bis(3-bromopropoxy)-26,28-dihydroxy-5 , 11,17,23-Tetra-tert-butylcalix[4]arene (Example 1, 2 g, 2.24 mmol), anhydrous potassium carbonate (4.03 g, 29.2 mmol). Serine ethyl ester hydrochloride 3.82 g (22.4 mmol), first add 1 / 3 of the amount, heat to reflux, then add 1 / 3 of the amount every 8 hours, and add the serine ethyl ester hydrochloride three times. After adding the last batch of serine ethyl ester hydrochloride, heat, stir and reflux for two days. After cooling, the solvent acetonitrile was evaporated to dryness under reduced pressure. Chloroform (100 ml) was added to the obtained solid, and washed three times with dilute hydrochloric acid, saturated aqueous sodium bicarbonate solution and distilled water respectively. Add 10 g of anhydrous sodium sulfat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com