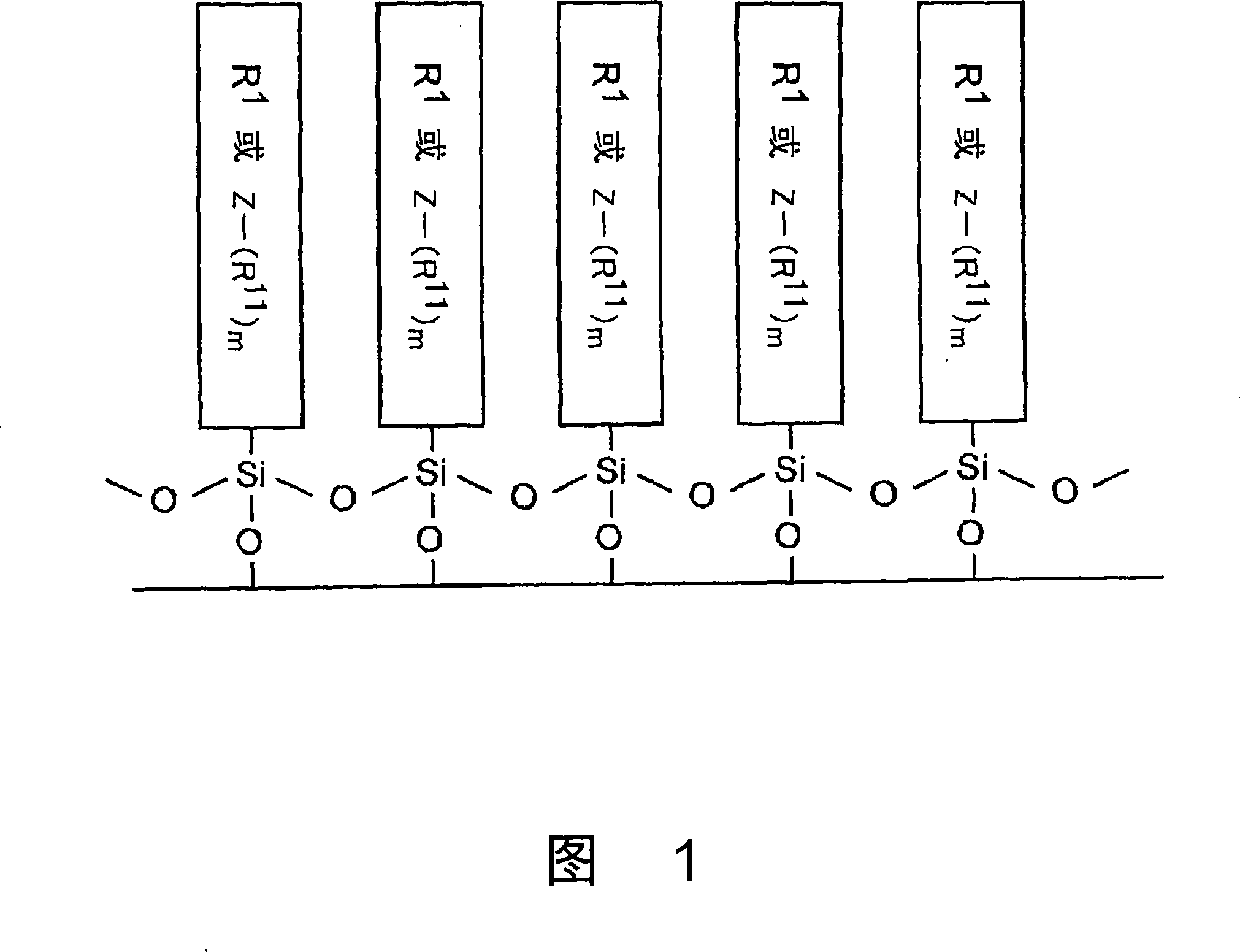
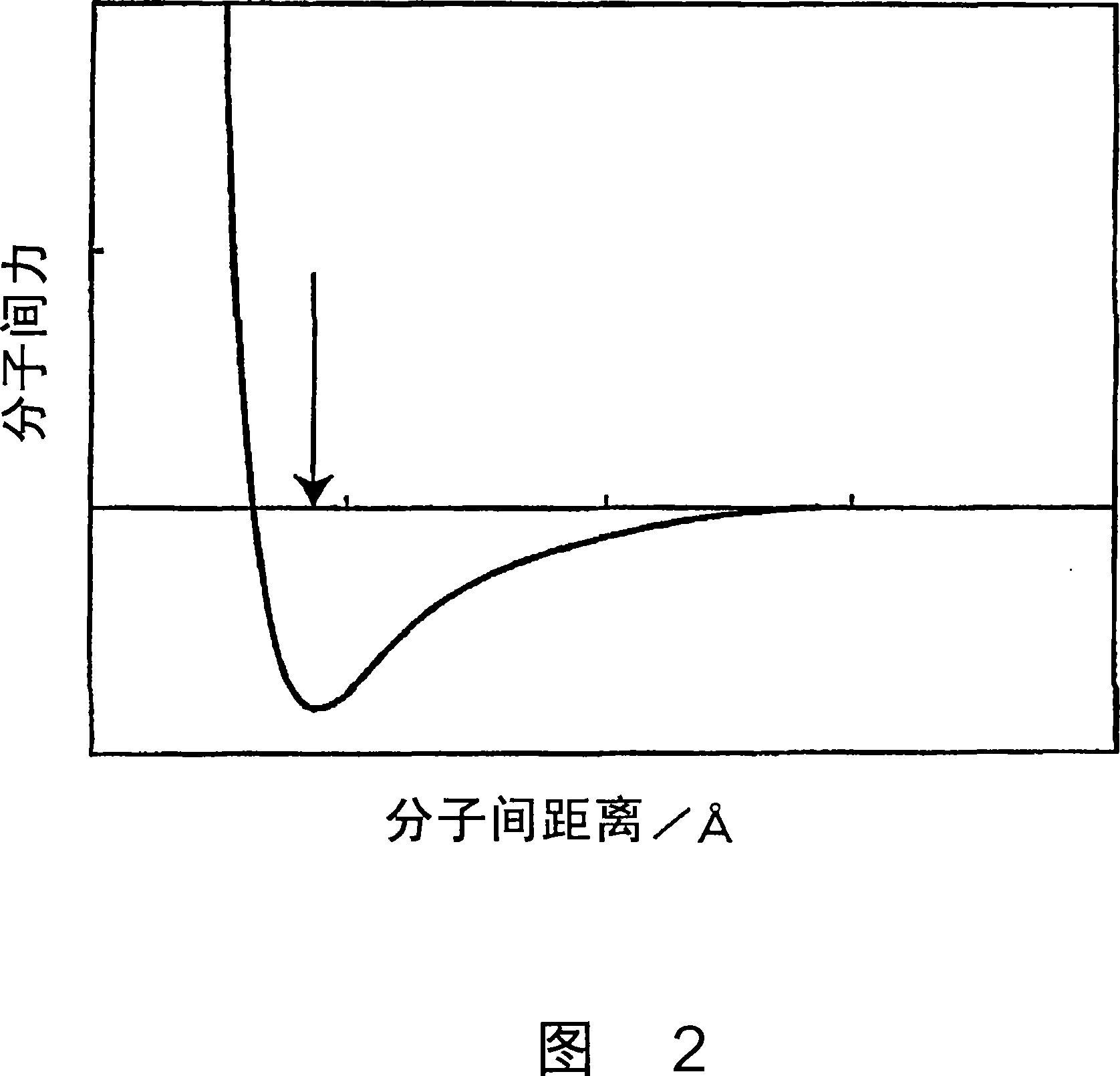
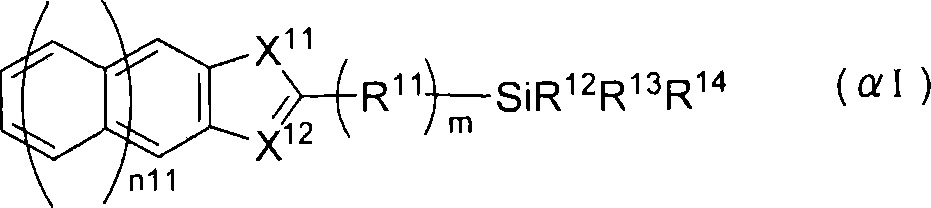P-electron conjugated organosilane compound and method for synthesizing same
An organosilane and compound technology, applied in the field of π-electron-conjugated organosilane compounds and their preparation, can solve the problems of difficulty in providing self-organized film conductivity, and achieve high stability, prevention of physical peeling, and high conductivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0314] Prepare three selenophene trichlorosilane (terselenophenetrichlorosilane) (X 1 =X 2 =X 3 = Cl; Y = Se; R 2 = R 3 =H and n1=3) (Grignard method)
[0315] This compound was prepared according to Synthetic Scheme 1. Specifically, at first, put 50ml chloroform and 70mM selenophene into a 100-ml round bottom flask, cool the mixture to 0°C temperature; add 20M NBS (N-bromosuccinimide) therein, stir The mixture was left for 1 hour. After the product was extracted with purified water, it was purified under reduced pressure at 80°C to obtain 2-bromoselenophene. Then, in a nitrogen atmosphere, 5 ml of anhydrous THF and 30 mM 2-bromoselenophene were put into a 50-ml round bottom flask; magnesium was added thereto; and the mixture was stirred for 2 hours. Then, add catalyst-containing Ni(dppp)Cl to it 2(dichloro[1,3-bis(diphenylphosphino)propane]nickel(II)) and 30 mM 2-bromoselenophene in 5 ml of anhydrous THF, and the mixture was reacted at 0°C for 12 hours. The product w...
preparation example 2
[0328] Preparation of tetraselenophene trimethoxysilane (X) represented by general formula (1) 1 =X 2 =X 3 =OCH 3 ;Y=Se,R 2 = R 3 = H; n1 = 4)
[0329] First, under a nitrogen atmosphere, 5 ml of anhydrous THF and 5 mM of the intermediate 2-bromodiselenophene obtained in Preparation Example 1 were put into a 50 ml round bottom flask; magnesium was added thereto; and the mixture was stirred for 2 hours. Then, add catalyst-containing Ni(dppp)Cl 2 and 5 mM 2-bromodiselenophene in 5 ml of anhydrous THF, and the compound was reacted at 0° C. for 10 hours. The product was purified by flash chromatography after extraction with purified water to obtain tetraselenophene (35%).
[0330] Then, 50 ml of chloroform and 70 mM of the intermediate tetraselenophene obtained in Preparation Example 2 were put into a 100-ml round bottom flask; the mixture was cooled to 0° C., and after adding 70 M NBS, stirred for 1 hour. After the product was extracted with purified water, it was purifie...
preparation example 3
[0340] Preparation of octaselenophene triethoxysilane (X) represented by general formula (1) 1 =X 2 =X 3 =OC 2 h 5 ;Y=Se;R 2 = R 3 = H; n1 = 8)
[0341] First, under a nitrogen atmosphere, 5 ml of anhydrous THF and 5 mM of the intermediate 2-bromotetraselenophene obtained in Preparation Example 2 were put into a 50 ml round bottom flask; magnesium was added thereto; and the mixture was stirred for 3 hours. Then, add catalyst-containing Ni(dppp)Cl 2 and 5 mM 2-bromotetraselenophene in 5 ml of anhydrous THF, and the compound was reacted at 0° C. for 12 hours. The product was purified by flash chromatography after extraction with purified water to obtain octaselenphene (30%).
[0342] Then, 50 ml of chloroform and 70 mM of the above octaselenphene were put into a 100-ml round bottom flask; the mixture was cooled to 0° C., and after adding 10 M NBS, the mixture was stirred for 1 hour. After the product was extracted with purified water, it was purified under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com