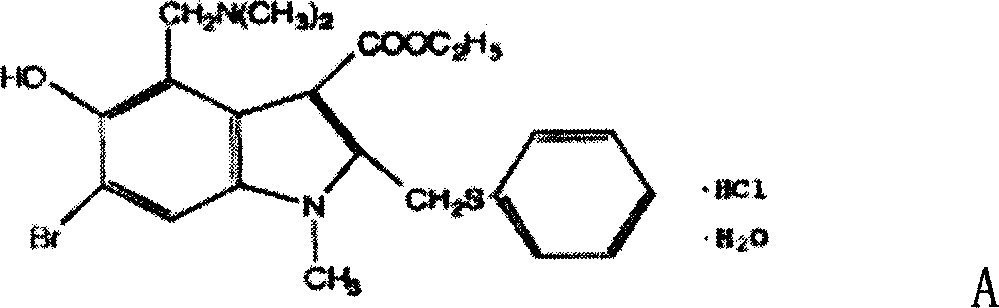
Use of medicine for treating virus infection of respiratory system
A technology for respiratory system and viral infection, applied in the direction of respiratory system diseases, antiviral agents, drug combinations, etc., can solve irreversible anemia, persistent incidence of respiratory system viral infection, leukopenia and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Arbidol hydrochloride is to the protective effect of infection coxsackie virus B3 animal
[0017] BalB / C mice, both male and female, were randomly divided into 6 groups: normal control group, virus control group, positive drug control group, test drug group (using 3 concentrations, namely Arbidol hydrochloride high-dose group, middle-dose group) and low dose group) 27 mice per group. Two days before the infection, the mice in each group of Arbidol hydrochloride were intragastrically administered, twice a day; the positive drug control group was injected intraperitoneally with ribavirin; 2 times. The normal control group was fed naturally without any treatment. On the day of the experiment, after routine disinfection of the infected parts of the mice, intraperitoneal injection of CVB 3 , Continue medication 2 hours after inoculation. Observe the survival rate; when continuous treatment reaches the acute stage, randomly kill 1 / 3 of each group, take the r...
Embodiment 2
[0019] Embodiment 2: Arbidol hydrochloride is to the protective effect of infection Coxsackie virus B5 animal
[0020] Balb / C mice, female, 5-7 weeks old, weighing 17-20g, were randomly divided into 5 groups: normal control group, virus control group, positive drug control group, treatment dose 25mg / kg / d experimental group and treatment Dose 50mg / kg / d experimental group, 10 rats in each group.
[0021] On the day of the experiment, after routine disinfection of the infected parts of the mice, intraperitoneal injection of CVB 5 . 24 hours after virus inoculation, the mice in each group of Arbidol hydrochloride were intragastrically administered, once a day, for 6 consecutive days; the normal control group did not receive any treatment; the virus control group was intragastrically administered with an equal volume of PBS to replace the drug . Six mice in each group were sacrificed on day 8 after virus inoculation. Parts of lung, heart and liver obtained from dissection were ...
Embodiment 3
[0029] Embodiment 3: Arbidol hydrochloride is to the protective effect of adenovirus infection animal
[0030] Mice were randomly divided into 6 groups, 10 mice in each group. They are: normal control group, virus control group, ribavirin group, Arbidol hydrochloride high, medium and low dose groups. The mice were anesthetized with ether, and then infected with adenovirus stock solution by intranasal instillation. After 4 hours, the mice in each group of Arbidol hydrochloride were intragastrically administered, twice a day, for 8 consecutive days; the virus control group was administered daily. The stomach was disinfected twice with drinking water; the normal control group received no treatment; the positive drug ribavirin control group received intraperitoneal injection of drugs. After 8 days of administration, the mice were killed and dissected, and their body weight was weighed with a precision balance; the blood was collected to separate the serum, and the anti-adenovirus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com