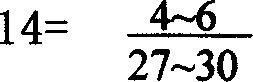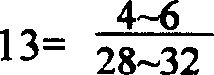Production method of soft capsule for treating pelvic inflammation
A production method and technology of soft capsules, which are applied in the field of drugs for treating pelvic inflammatory disease, can solve the problems of undisclosed content and drug preparation methods, etc., and achieve the purpose of inhibiting the increase of abdominal capillary permeability, improving the pain threshold of hot plate reaction, and inhibiting The effect of the number of twists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Weigh the following traditional Chinese medicine ingredients (unit: g)
[0077] Golden cherry root 18.8; Millipeda 32.7; Qianjinba 18.1; Gongluomu 18.3; LMZ 7.5;
[0078] Its production steps are:
[0079] (1) Take 2.1 g of the total amount of Andrographis paniculata, pulverize it into fine powder with superfine pulverization technology, and sterilize it with high-pressure steam at 100-120° C. for 30-60 minutes to obtain intermediate product A;
[0080] (2) Get remaining Andrographis paniculata 4.5g soak 1 hour with the ethanol of volume concentration 60-80%, carry out reflux alcohol extraction, the solution after alcohol extraction reclaims ethanol, obtains alcohol extraction part B;
[0081] (3) Gongluomu and Liangmianzhen were decocted twice, each time for 2 to 3 hours, filtered the medicinal solution, combined with the extracted solution twice, and concentrated to obtain the water-extracted part C;
[0082] (4) Boil Rosa japonica root, Spatholobus Spatholobus, and...
Embodiment 2
[0086] Weigh the following traditional Chinese medicine ingredients (unit: g)
[0087] Golden Cherry Root 8, Millipede 15, Qianjinba 8, Gonggongmu 8, LMZ 3, Andrographis 2.5;
[0088] Its production steps are:
[0089] (1) Take 0.8 g of the total amount of Andrographis paniculata, pulverize it into fine powder with superfine pulverization technology, and sterilize it with high-pressure steam at 100-120°C for 30-60 minutes to obtain intermediate product A;
[0090] (2) Get 1.7 parts by weight of the remaining Andrographis paniculata and soak it in ethanol with a volume concentration of 60-80% for 1 hour, carry out reflux alcohol extraction, and recover ethanol from the solution after alcohol extraction to obtain alcohol extraction part B;
[0091] (3) Gongluomu and Liangmianzhen were decocted twice, each time for 2 to 3 hours, filtered the medicinal solution, combined with the extracted solution twice, and concentrated to obtain the water-extracted part C;
[0092] (4) Boil R...
Embodiment 3
[0096] Weigh the following traditional Chinese medicine ingredients (unit: g)
[0097] Golden cherry root 23.4; Millipeda 38.8; Qianjinba 22.9; Gongluomu 23.2;
[0098] Its production steps are:
[0099] (1) Take 3 g of the total amount of Andrographis paniculata, grind it into fine powder, and sterilize it with high-pressure steam at 100-120° C. for 30-60 minutes to obtain intermediate product A;
[0100] (2) Take the remaining 5.1g of Andrographis paniculata, together with the weighed golden cherry root, Caulis Spatholobus, Qianjinba, Gongluomu, and Liangmianzhen, add 5 to 8 times the amount of water, decoct twice, each time for 2 to 3 hours, medicine Liquid was filtered, and the two boiling extracts were combined to obtain the water extraction part B;
[0101] (3) Concentrate B, dry it, and pulverize it into a fine powder to obtain the intermediate product dry cream powder C;
[0102] (4) Mix the intermediate products A and C, granulate, mix with appropriate amount of au...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Severe | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com