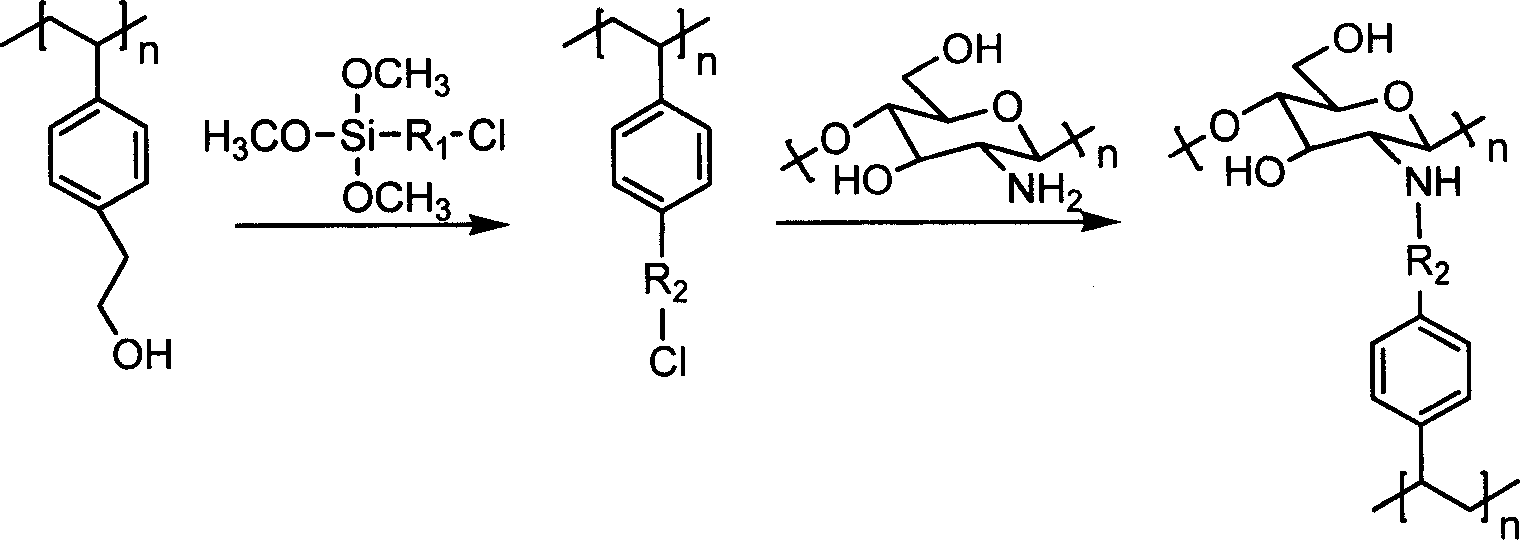
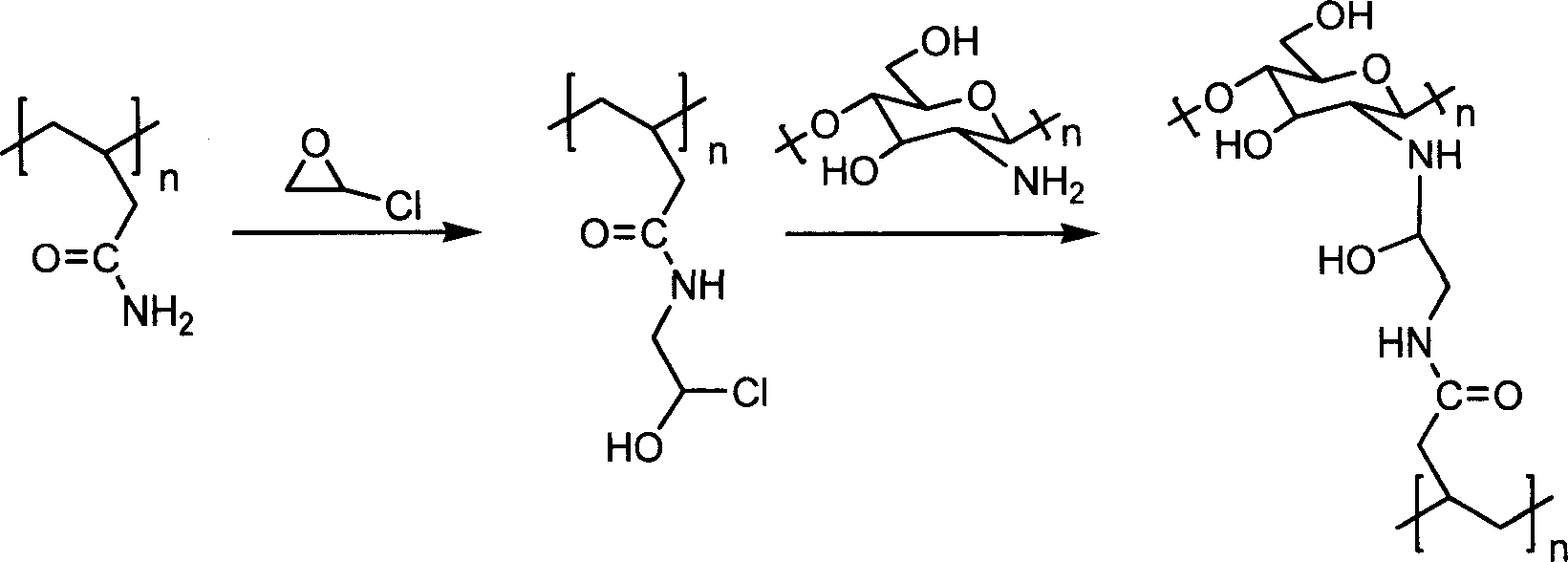
Penicillin acylation enzyme-fixing carrier preparation method and carrying method
A penicillin acylase, carrier technology, applied in the direction of immobilization on/in organic carriers, hydrolase, etc., can solve the problems of unsatisfactory mechanical properties and unsuitable reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 5.0 g of polymethyl methacrylate spherical porous resin was ground and sieved to obtain resin particles with a particle size of 0.5 mm. Dissolve 1.0 g of chitosan in 50 ml of 2% acetic acid solution, and then add 0.5 g of PEG20000 to form a transparent and clear solution. Mix the resin and chitosan solution evenly, and dry at 40°C. Transfer the mixed solids into a round-bottomed flask, add 50ml of water, and stir mechanically at 200r / min. After 30 minutes, adjust the pH value to 10 with NaOH aqueous solution, add 0.5g of 2-chloroethylamine hydrochloride, and control the temperature at 40°C. After reacting for 5 hours, filter with suction, wash, and dry at 40°C.
[0045] Take 1.0 g of the prepared carrier in a Erlenmeyer flask, add 1 mol / L sodium bicarbonate solution, react at 50°C for 12 hours, separate, then add 10 ml of 2.0% glyoxal solution, shake on a shaking table, 200r / min, the temperature is controlled at 40°C. Take it out after 6 hours, age at 30°C for 24 ho...
Embodiment 2
[0047] 20.0 g of poly(p-styrene alkylhydroxyl) spherical porous resin was ground and sieved to obtain resin particles with a particle size of 0.5 mm. Dissolve 1.0 g of chitosan in 50 ml of 1% hydrochloric acid solution, and then add 0.5 g of PEG2000 to form a transparent and clear solution. Mix the resin with the chitosan solution and dry at 40°C. Transfer the mixed solids into a round bottom flask, add 50ml DMSO, and after 30 minutes, mechanically stir at 500r / min, adjust the pH value to 9 with NaOH aqueous solution, add 5ml of trimethoxychloromethylsilane, control the temperature at 60°C, and react After 4 hours, filter with suction, wash, and dry at 40°C.
[0048] Take 1.0 g of the prepared carrier in an Erlenmeyer flask, add 1 mol / L ammonia water, react at 60°C for 15 hours, separate, then add 10 ml of 2.0% glutaraldehyde solution, vibrate on a shaker, 200r / min, temperature Controlled at 40°C. Take it out after 4 hours, age at 30°C for 36 hours, add free penicillin acyl...
Embodiment 3
[0050] 10.0 g of polyacrylic acid tertiary amino porous resin was ground and sieved to obtain resin particles with a particle size of 0.5 mm. Dissolve 1.0 g of chitosan in 50 ml of 1% hydrochloric acid solution, then add 0.5 g of glucose to form a transparent and clear solution. Mix the resin and chitosan solution evenly, and dry at 40°C. Transfer the mixed solids into a round bottom flask, add 25ml DMF and 25ml ethanol, after 30 minutes, mechanically stir at 500r / min, adjust the pH value to 11 with NaOH aqueous solution, add 5ml of epichlorohydrin, control the temperature at 90°C, and react After 8 hours, filter with suction, wash, and dry at 50°C.
[0051] Take 1.0g of the prepared carrier in a Erlenmeyer flask, add 1mol / L sodium polyphosphate solution, react at 30°C for 12 hours, separate, then add 10ml of 2.0% glyoxal solution, oscillate on a shaking table, 200r / min, the temperature is controlled at 40°C. Take it out after 6 hours, age at 30°C for 48 hours, add free pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com