Polypyrrole electrolytic synthesis method
A technology for electrolytic synthesis and polypyrrole, which is applied in electrolytic processes, electrolytic components, electrolytic organic production, etc., can solve problems such as low catalytic activity, and achieve the effects of low preparation cost, good application and development prospects, and good electrocatalytic oxidation characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
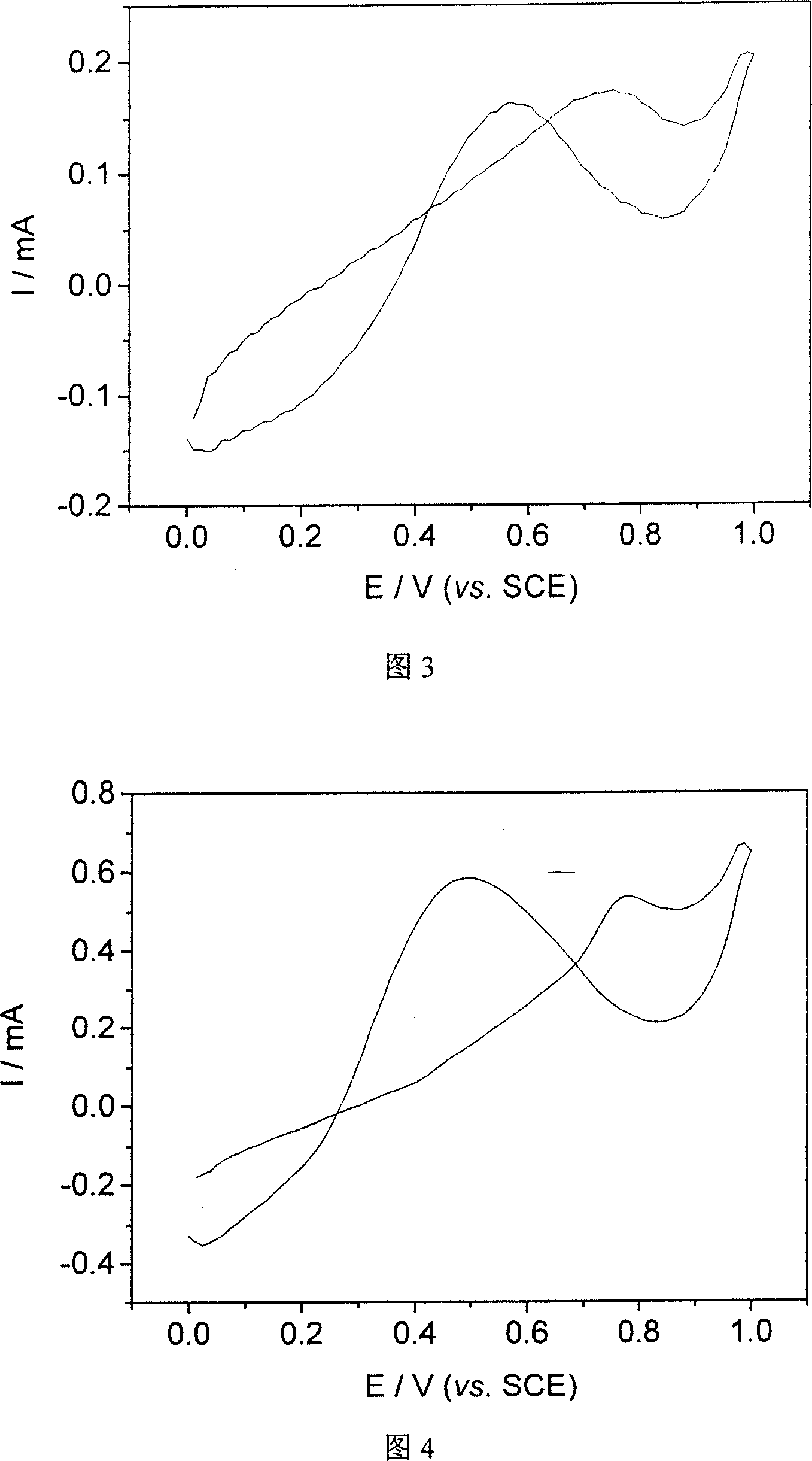
[0022] The raw material for polymerization monomer is pyrrole, the concentration is 0.4mol / L, and pyrrole is purified by distillation before the experiment. 1-Ethylimidazole trifluoroacetate acts as both solvent and electrolyte. The platinum electrode is the working electrode. Before the experiment, the platinum electrode needs to be burned to remove the impurities adsorbed on the surface, and then ultrasonically cleaned with acetone and double distilled water in turn; the large-area platinum sheet is both a reference electrode and an auxiliary electrode. Polypyrrole was prepared by cyclic voltammetry, the scanning speed was 50mV / s, and the scanning potential range was 0-1.2V. The prepared polypyrrole was rinsed with distilled water, and dried in a vacuum oven at 40° C. to obtain a dark brown polypyrrole conductive polymer.
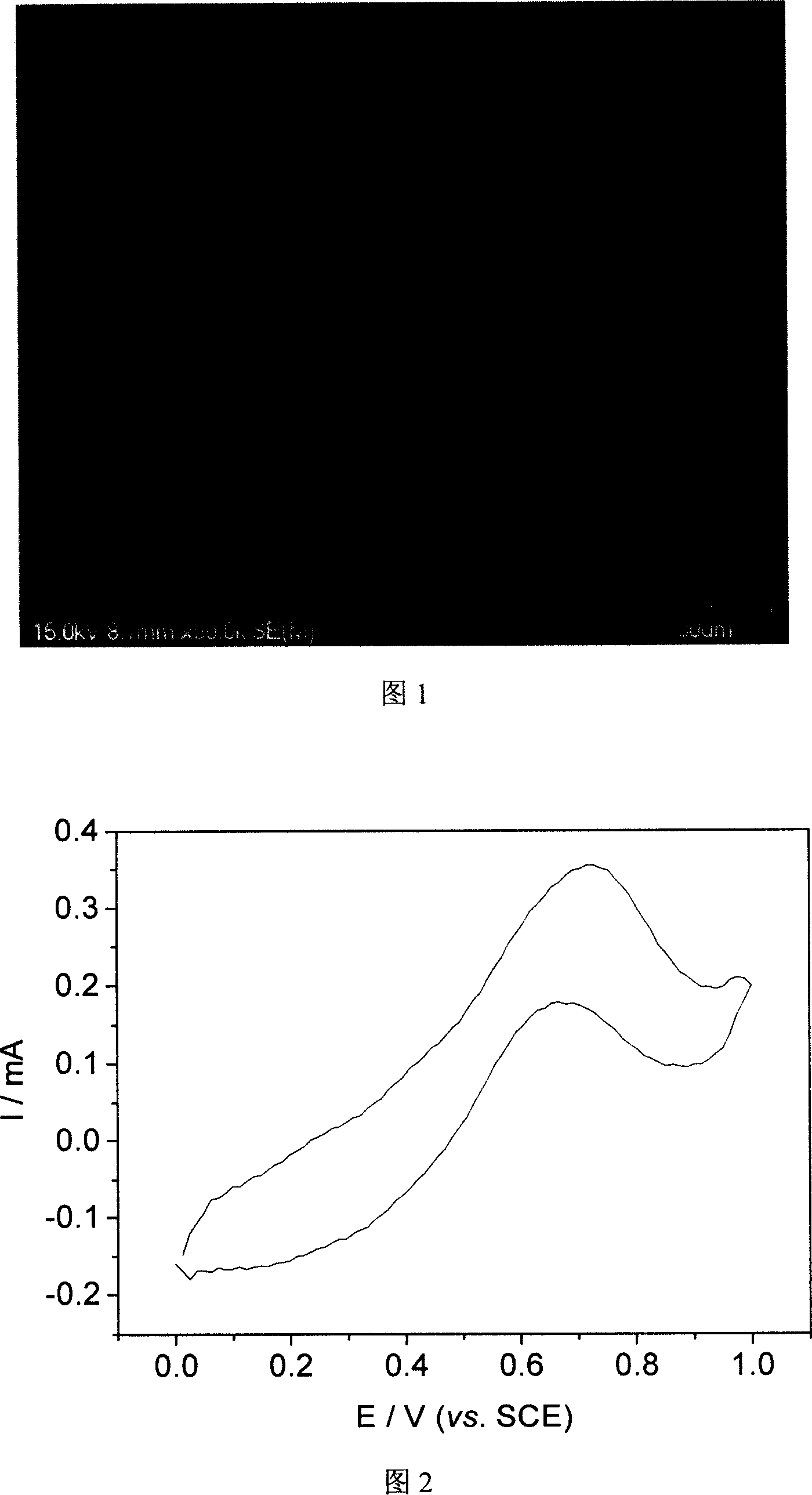
[0023] Referring to FIG. 1 , it is found through scanning electron microscopy that the structure of polypyrrole is very flat and regular, consisting of ...
Embodiment 2
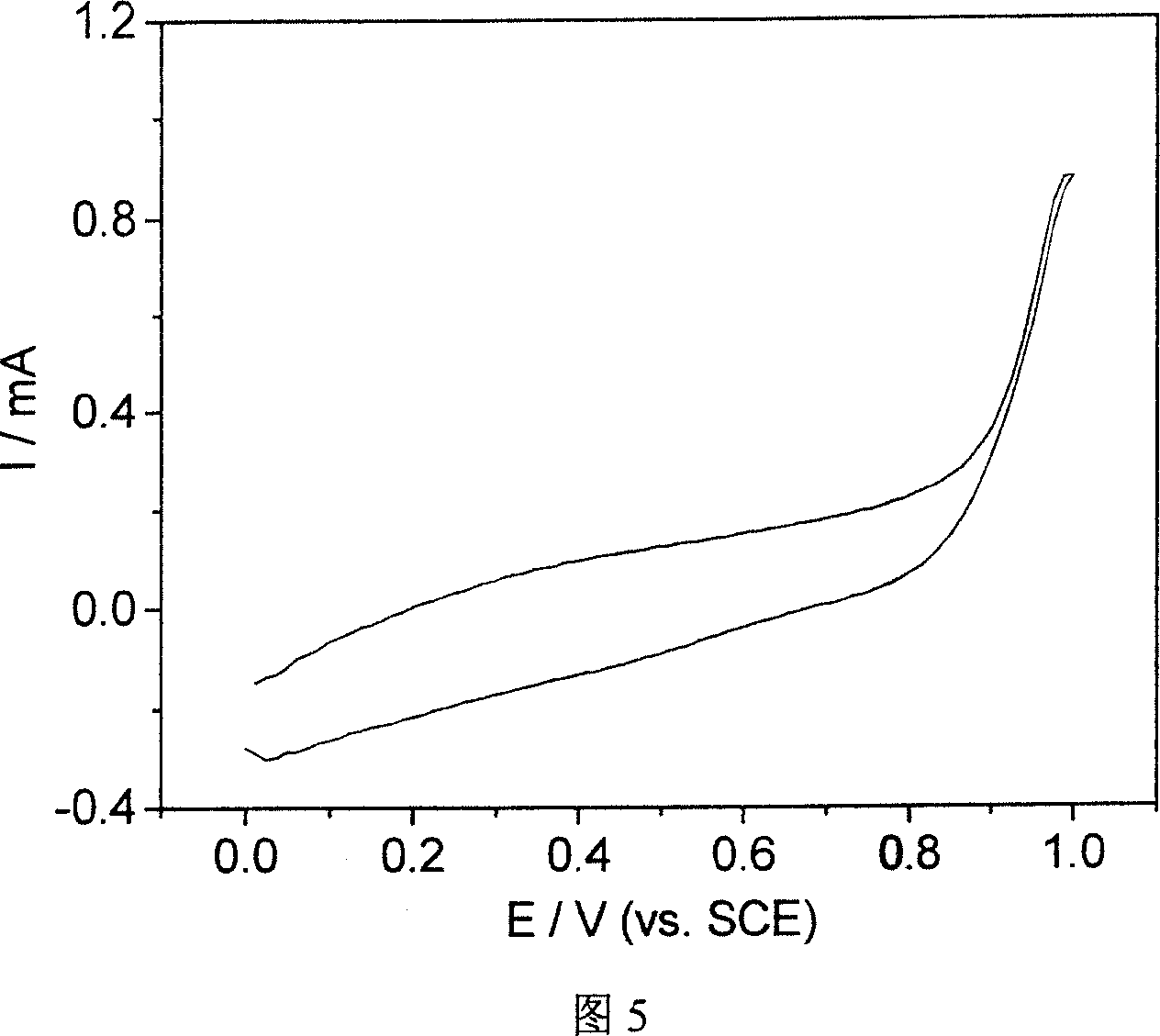
[0026] The monomer raw material for polymerization is pyrrole, the concentration is 0.3mol / L, and pyrrole is purified by distillation before the experiment. 1-Ethylimidazole trifluoroacetate acts as both solvent and electrolyte. The platinum electrode is the working electrode. Before the experiment, the platinum electrode needs to be burned to remove the impurities adsorbed on the surface, and then ultrasonically cleaned with acetone and double distilled water in turn; the large-area platinum sheet is both a reference electrode and an auxiliary electrode. Polypyrrole was prepared by constant current method, and the polymerization current density was 0.5mA / cm 2 . The prepared polypyrrole was rinsed with distilled water, and dried in a vacuum oven at 50° C. to obtain a dark brown polypyrrole conductive polymer.
[0027] Figure 3 illustrates that the polypyrrole electrode synthesized under the conditions of this example has better electrocatalytic activity to ethanol. When sca...
Embodiment 3
[0029] The raw material for polymerization monomer is pyrrole, the concentration is 0.4mol / L, and pyrrole is purified by distillation before the experiment. 1-Ethylimidazole trifluoroacetate acts as both solvent and electrolyte. The platinum electrode was used as the working electrode. Before the experiment, the platinum electrode was burned to remove the impurities adsorbed on the surface, and then ultrasonically cleaned with acetone and double-distilled water in sequence; the saturated calomel electrode was used as the reference electrode, and the large-area platinum sheet was used as the auxiliary electrode. Polypyrrole was prepared by constant potential electrolysis, and the polymerization potential was 1.0V. The prepared polypyrrole was rinsed with distilled water, and dried in a vacuum oven at 40° C. to obtain a dark brown polypyrrole conductive polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com