Recombinant virus vectors
a virus vector and recombinant technology, applied in the field of mutation viruses, can solve the problems of inacceptable use of an unmodified form of an hsv virus vector, cell damage or cell death, and disruption of the normal activities of cells, so as to minimise the risk of reversion, reduce or zero risk, and minimise the risk of regenerating wild type virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
)
[0041] To illustrate further the present invention and how to carry it out, a preferred embodiment is described below, with reference to accompanying drawings, given by way of example only and not by way of limitation.
[0042] In the accompanying drawings:
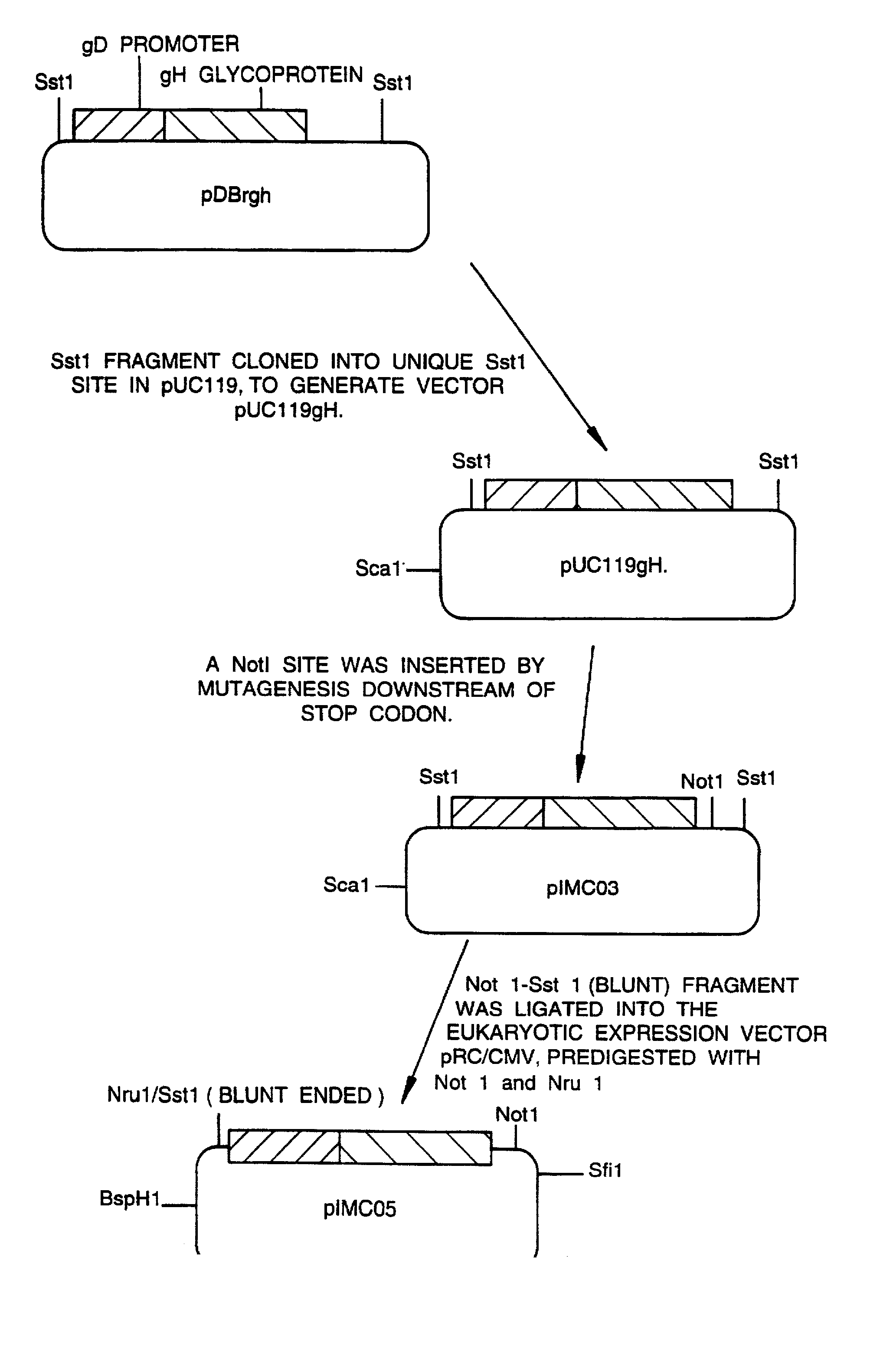
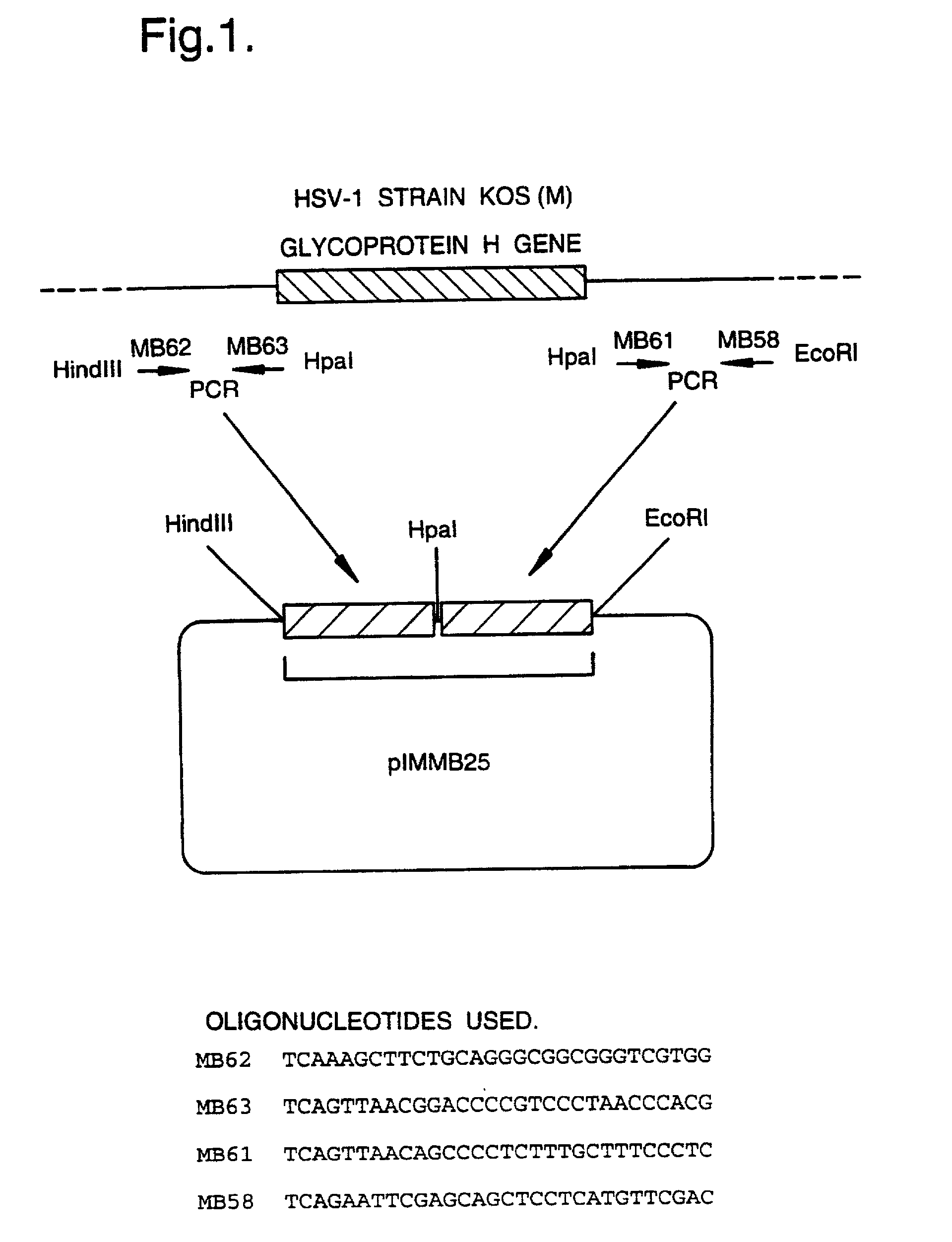
[0043] FIG. 1 shows the construction of a plasmid pIMMB25;
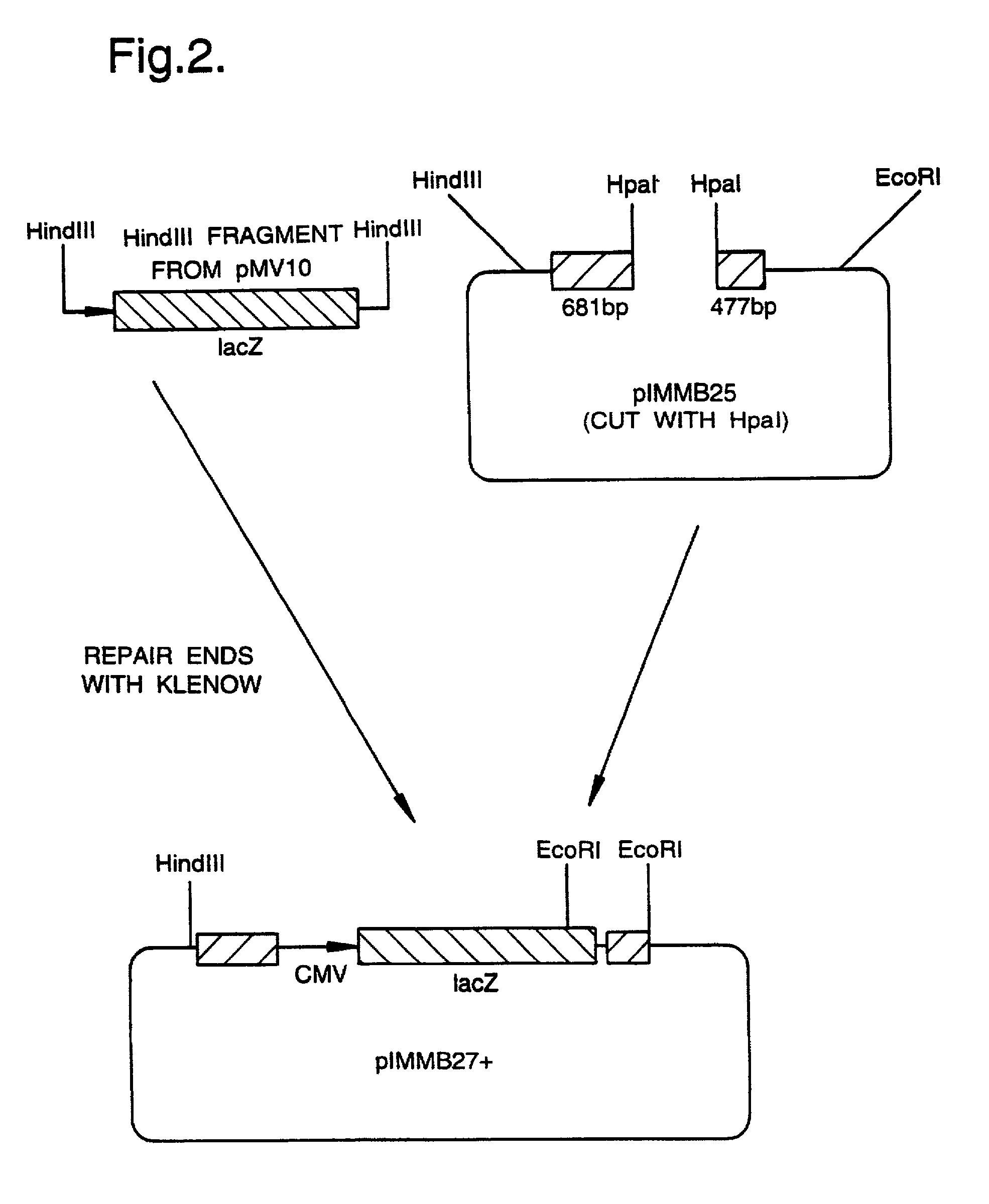
[0044] FIG. 2 shows the construction of a plasmid pIMMB27+;
[0045] FIG. 3 shows the construction of a plasmid pIMC05.
[0046] All genetic manipulation procedures mentioned herein are carried out according to standard methods described in `Molecular Cloning, A Laboratory Manual`, eds. Sambrook, Fritsch and Maniatis, Cold Spring Harbor Laboratory Press, 1989, incorporated by reference. The example below refers to the construction and properties of a gH-defective virus as described in WO 92 / 05263 and in A Forrester et al, J Virol 66(1), 1992, pp 341-348.
[0047] The virus construct described in WO 92 / 05263 and in A Forrester et al, J Virol 66(1) 1992, pp 341-348, contains a deletion ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| genetic defect | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com