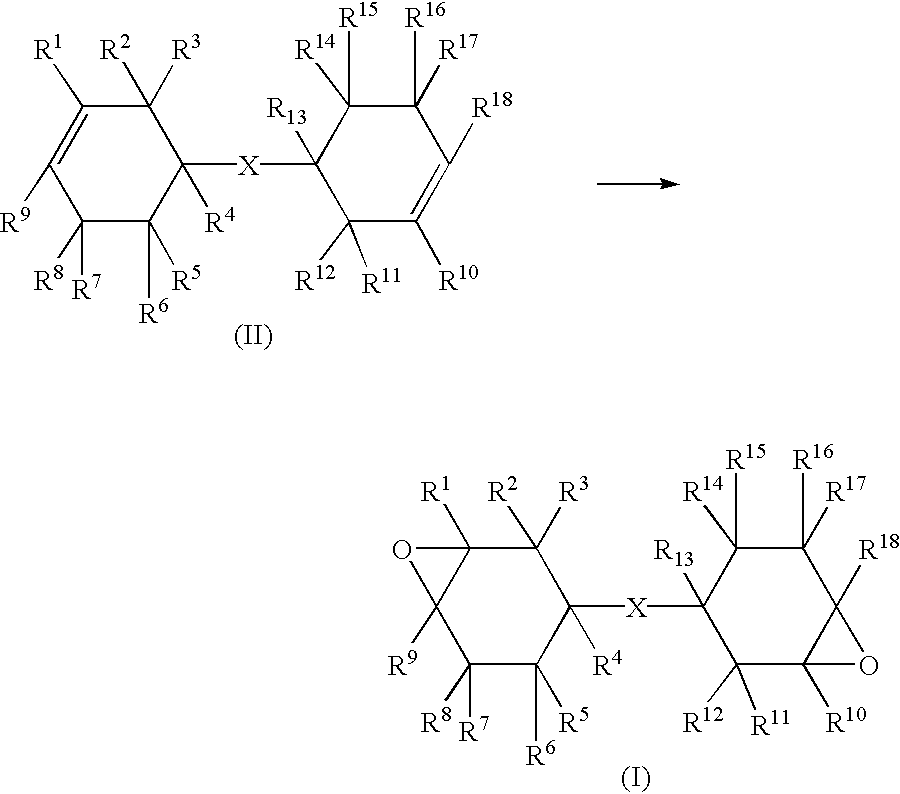
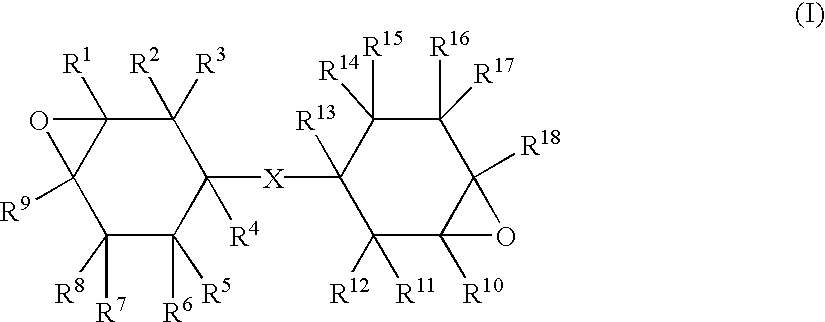
Method of producing epoxy compound, epoxy resin composition and its applications, ultraviolet rays-curable can-coating composition and method of producing coated metal can
a technology of epoxy resin and composition, applied in the direction of synthetic resin layered products, instruments, photomechanical devices, etc., can solve the problems of insufficient adhesion to a raw material and processability, poor surface curability, insufficient film performance, etc., to achieve excellent moisture and heat resistance and transparency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
production example 1
[0185] 10% acetaldehyde-ethyl acetate solution containing cobalt acetate was charged in a 300 ml stainless reactor equipped with an air inlet, a perforated plate for dispersing a gas and a cooling jacket at 114 kg / h, and reaction was conducted at 45.degree. C. while blowing compressed air. The resulting reaction solution contained 10.1% of peracetic acid, 2.2% of acetaldehyde monoperacetate and 2.0% of acetic acid. This solution was charged in a distillation column together with sodium polyphosphate, followed by concentration, thereby obtaining a peracetic acid solution. In the solution, a peracetic acid concentration was 29.1% and water content was 0.47%.
example i-1
[0186] Synthesis of Alicyclic Epoxy Compound (A-1)
[0187] 36 g of water, 12.0 g of sodium hydrogensulfate, 500 g of isopropylidene-4,4'-dicyclohexanol (a product of Aldrich Chemical Company Inc.) and 500 g of Solvesso 150 (a product of Exxon Chemical) as a solvent were added to a 1 liter flask with a jacket, which is equipped with a stirrer, a cooling tube, a thermometer and a nitrogen inlet, and dehydration reaction was conducted at 100.degree. C.
[0188] Reaction was completed at the time when water was no longer distilled off. As a result of analysis of the reaction liquid with gas chromatography, 2,2-bis (3',4'-cyclohexenyl)propane was formed in a yield of 96%. The reaction solution obtained was washed with 500 ml of ion-exchanged water using a separatory funnel. An organic layer was then distilled under reduced pressure to obtain 387.0 g of colorless, transparent liquid 2,2-bis(3',-4'-cyclohexenyl)propane. Purity of the liquid obtained was 96.1%.
[0189] 100 g of the 2,2-bis(3'-4'-c...
example i-2
[0191] Synthesis of Alicyclic Epoxy Compound (A-2)
[0192] 300 g of 4,4'-dicyclohexanolmethane, 600 g of toluene, 3 g of paratoluenesulfonic acid were added to a 1 liter flask with a jacket, which is equipped with a stirrer, a cooling tube, a thermometer and a nitrogen inlet, and dehydration reaction was conducted at 110.degree. C.
[0193] Reaction was completed at the time when water was no longer distilled off. As a result of analysis of the reaction liquid with gas chromatography, di(3,4-cyclohexenyl)methane was formed in a yield of 96%. The reaction solution obtained was washed with 500 ml of ion-exchanged water using a separatory funnel. An organic layer was then distilled under reduced pressure to obtain 269 g of colorless, transparent liquid di(3-4-cyclohexenyl)methane.
[0194] 100 g of the di(3-4-cyclohexenyl)methane and 200 g of ethyl acetate were charged in the same type of a 1 liter flask with a jacket as in Example I-1. While blowing nitrogen to a gas phase, 276.2 g of an ethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com