Textile care composition
a technology of textile care and composition, applied in the field of textile care composition, can solve the problems of textile surface appearance, textiles can be damaged in several ways, and the mechanical and/or chemical treatment of textiles is not fair enough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0120] Method of Preparation of Voranol Iso-Thiouronium Compounds
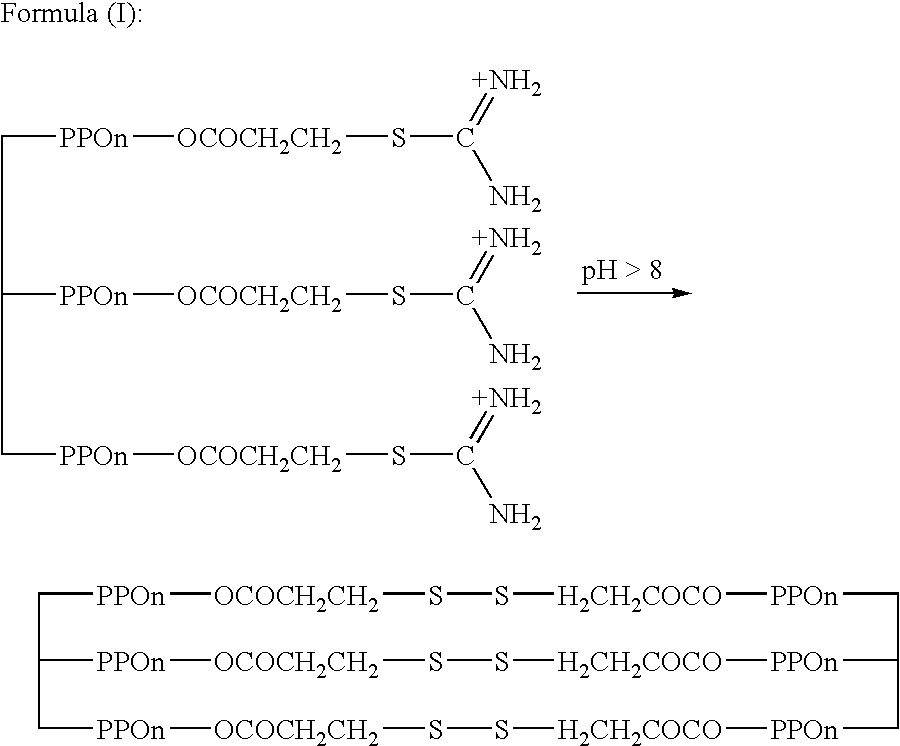
[0121] Voranol CP3055 .TM. (ex-Dow Chemicals) (29.4 g, 0.01 moles, mw 2940) was dissolved in toluene 50 mls. To this was added 3-bromopropionic acid (4.95 g, 3.3 equivalents ex Aldrich) and p-toluene sulphonic acid (1 g) to act as an acid catalyst. The solution was stirred by means of a magnetic follower on a hot plate. The solution was refluxed with a Dean and Stark distillation trap. After 1.5 hours no more water was seen to azeotrope from the reaction vessel into the side arm. The reaction was stopped and allowed to cool.
[0122] The toluene / Voranol solution was shaken with solution of sodium bicarbonate (10 g / l). This was repeated five times, until the solution was seen not to effervesce from the evolution of carbon dioxide gas and the water was at a neutral pH. The two phases were separated and the toluene was removed from the Voranol compound by rotary evaporation. After drying in vacuo over calcium chloride the yi...
example 2
[0129] Method of Preparation of Jeffamine Iso-Thiouronium Compounds
[0130] Jeffamine T-3000 .TM. (ex-Huntsman Corp) (30 g, 0.01 m mw.about.3000) was dissolved in acetone (50 mls) and cooled to below 5.degree. C. with an ice bath. Sodium carbonate (2 g) was added to the solution with stirring. 3-Chloropropionyl chloride (4.19 g 3.3 equivalents) was dripped into the solution of Jeffamine T-3000 over a period of one hour. The temperature of the solution was kept below 5.degree. C. After the addition of the acid chloride, the temperature was allowed to rise to room temperature over a period of one hour. The solution was then filtered to remove the solid sodium carbonate. The acetone was removed on a rotary evaporator. The solution was then washed five times with a sodium bicarbonate solution (10 g / l.sup.-1) until the washings were neutral. The product was then separated from the aqueous solution using a separating funnel and dried over silica in vacuo.
[0131] Yield 26.21 g (theoretical 33...
example 3
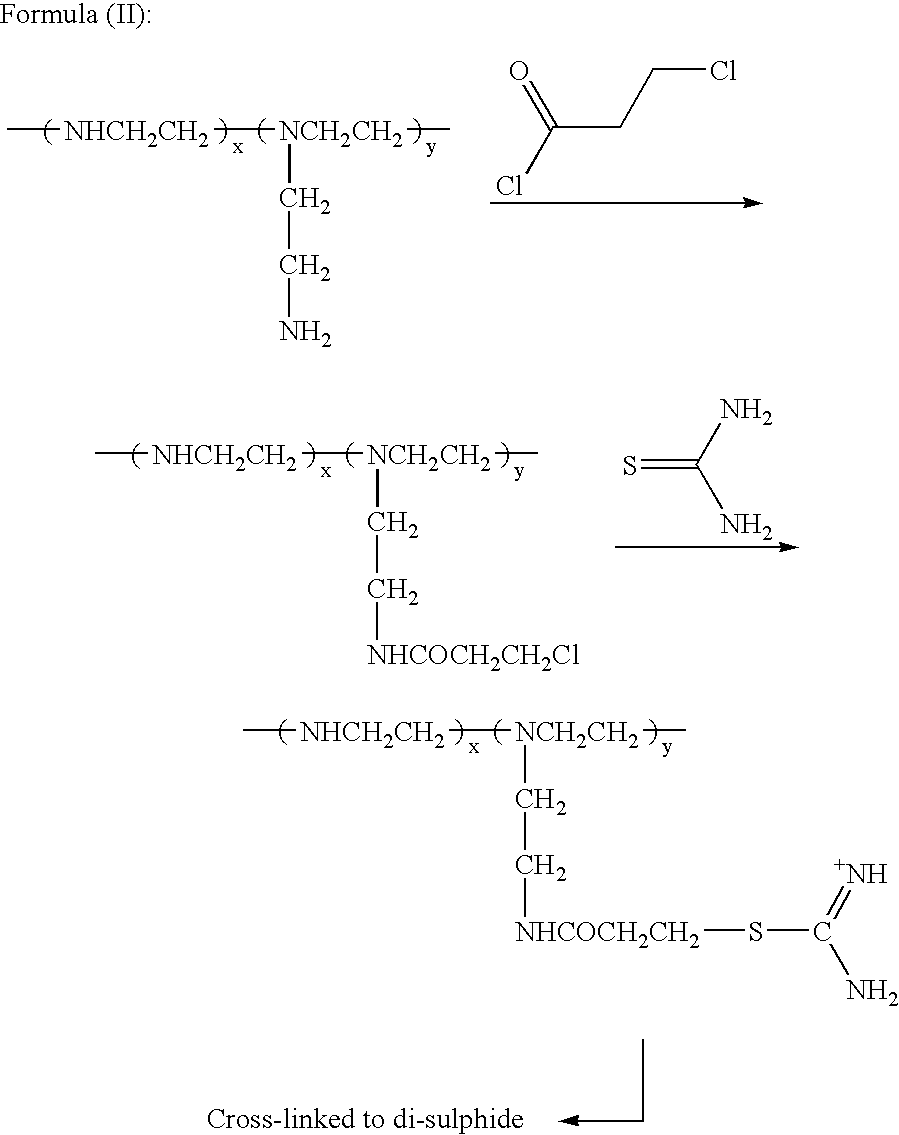
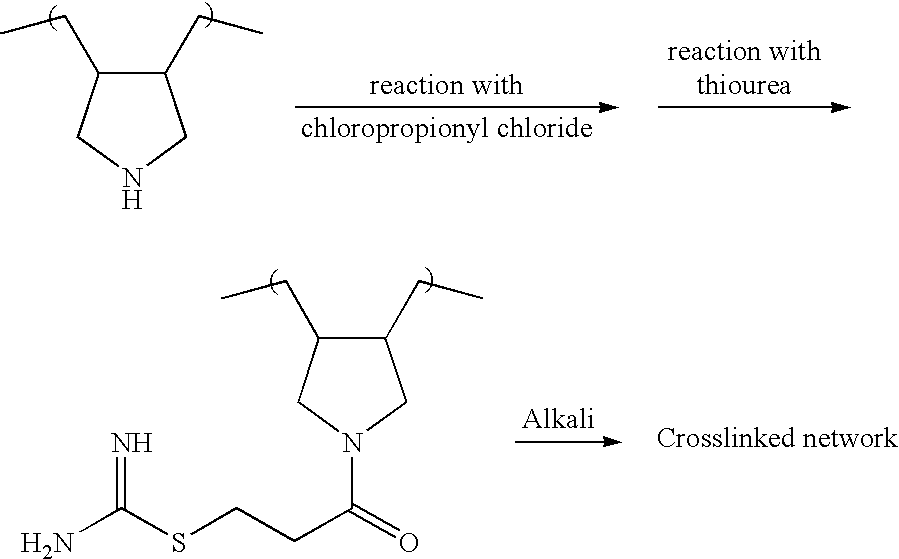
[0138] Method of Preparation of Lupasol Isothiouronium Compounds
[0139] Lupasol G-20.TM. (ex-BASF) (39 g 0.03 moles was dissolved in ethanol (50 mls) and cooled to below 5.degree. C. with an ice bath.
[0140] Sodium carbonate (3 g) was added to the solution with stirring. 3-Chloropropionyl chloride (14.22 g, 0.09 moles, 3 equivalents) was dripped into the Lupasol solution over a period of two hours. The temperature of the solution was kept below 5.degree. C. After the addition of the acid chloride, the temperature was allowed to rise to room temperature over a period of one hour. The solution was then filtered to remove the solid sodium carbonate. The product was not isolated using the methods described previously since the compound is highly water soluble and recovery from an aqueous solution would haven proven difficult. A small quantity was isolated for structure confirmation. A new peak was seen at 1637 cm.sup.-1, indicating the presence of an amide group.
[0141] To the alkylated Lu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com