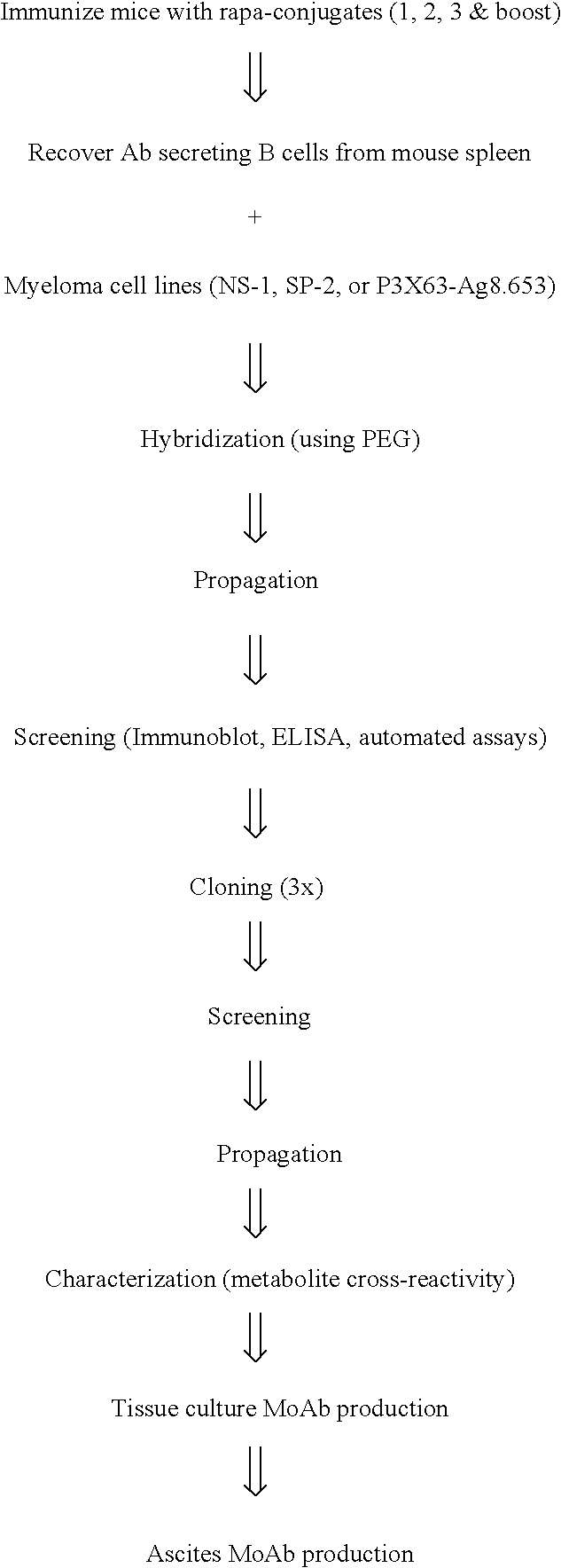
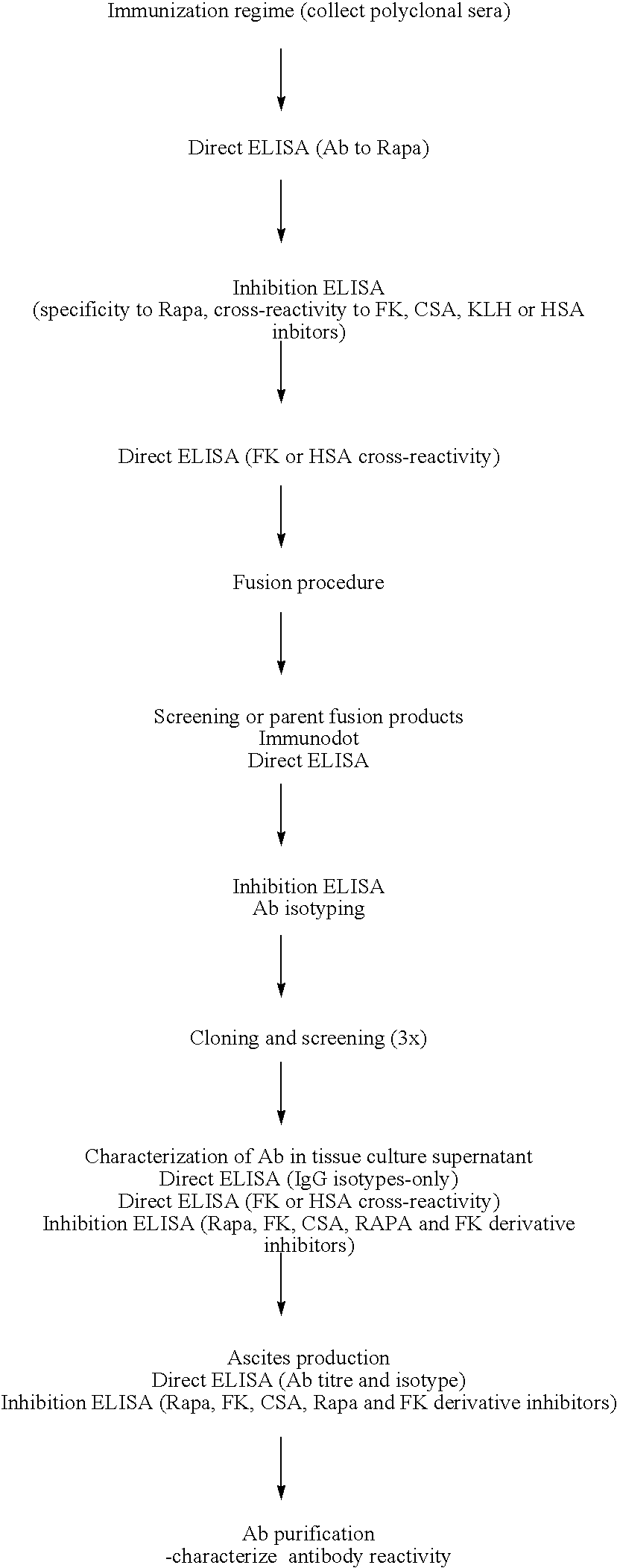
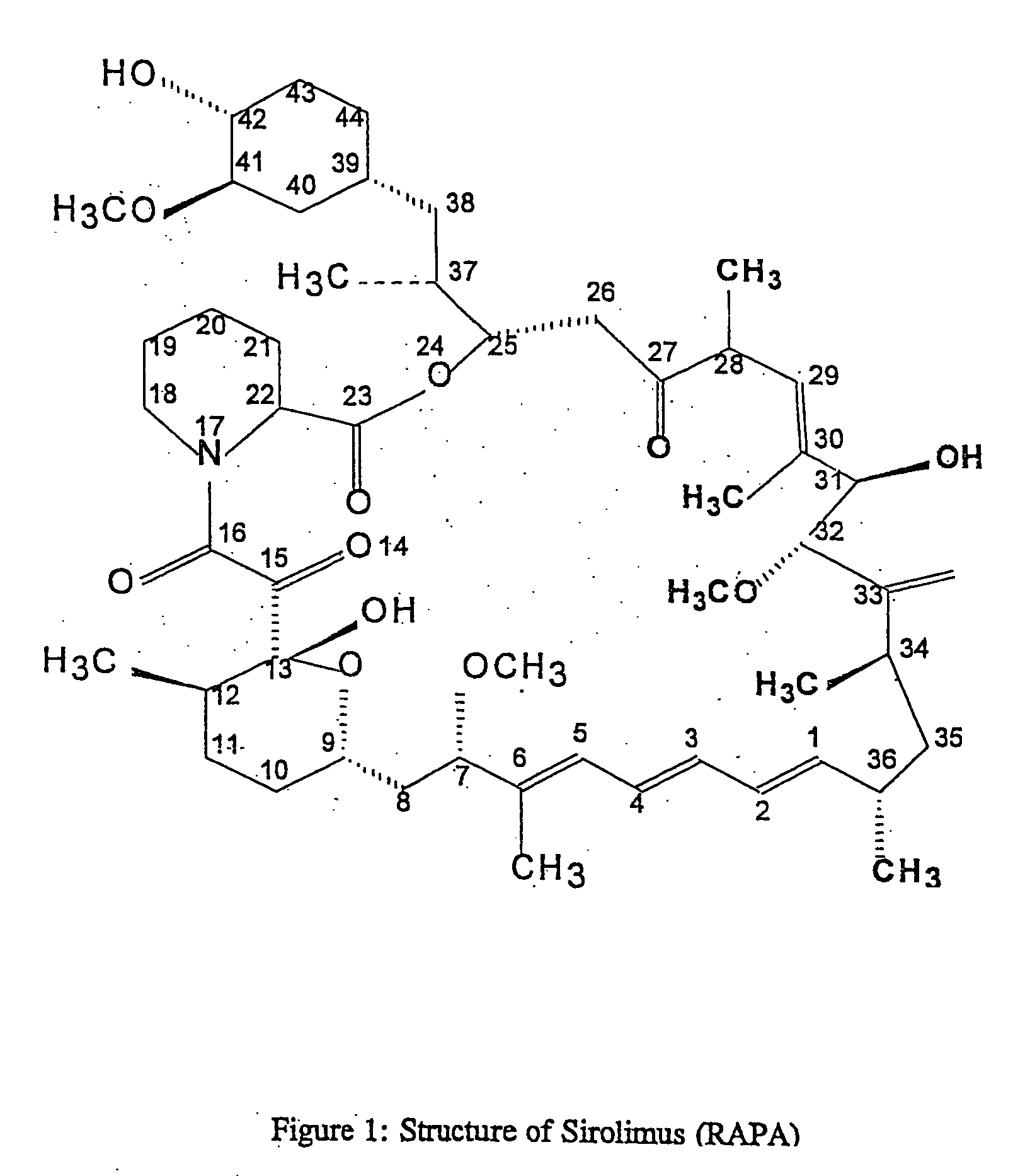
Method for producing rapamycin-specific antibodies
a technology of rapamycin and specific antibodies, which is applied in the field of producing rapamycin-specific antibodies, can solve the problems of not being able to establish a consensus regarding the identity or steady state concentration of whole blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of Rapamycin-42-succinate and Conjugation to a Protein Carrier
[0021] Preparation of rapamycin-42-O-hemisuccinate: Dimethylaminopyridine (11.8 mg, 97 .mu.mol) was added to a solution of rapamycin (80.0 mg, 88 .mu.mol) and succine anhydride (30.7 mg, 307 .mu.mol) in 2 mL dry pyridine and the mixture stirred at room temperature for 23 hours. The pyridine was evaporated and the residue dissolved in ethyl acetate. The ethyl acetate solution was washed twice with water and finally with brine before drying over magnesium sulfate and evaporating the solvent. The residue was eluted through a silica gel column using methanol / chloroform (1:19) and then methanol / chloroform (1:9) as eluent to give 20.0 mg (23%) of the product as a colorless solid.
[0022] Preparation of 42-O-succinimidooxysuccinyl rapamycin: N-hydroxysuccinimide (2.3 mg 19.7 .mu.mol) was added to a solution of rapamycin-42-0-hemisuccinate (20.0 mg, 19.7 .mu.mol) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) (...
example 3
Synthesis of Rapamycin-27-Oxime-Divinyl Sulfone and Conjugation to a Protein Carrier
[0025] Preparation of rapamycin-27-oxime: Hydroxylamine hydrochloride (3.0 mg, 44 .mu.mol) in 100 mL of water was added to a solution of rapamycin (20.0 mg, 22 .mu.mol) and pyridine (40 mL) in 4 mL of ethanol and the reaction mixture stirred at room temperature for 24 hours. The reaction mixture was diluted with ethyl acetate and washed sequentially with water, dilute aqueous hydrochloric acid, and brine. The organic phase was dried over magnesium sulfate and the solvent evaporated to give 20 mg of crude product.
[0026] Analysis of rapamycin-27-oxime-divinyl: LC / MS analysis (Gradient condition: 25 / 25 / 50 water / acetonitrile / methanol at 0 minutes up to 20 / 30 / 50 water / acetonitrile / methanol at 18 minutes. Column: SPHERISORB.TM. C-8 (octyl bonded spherical silica packing, Waters) semi-prep. Temperature was 35.degree. C. and the flow rate set at 3.5 mL / min. The UV signal was monitored at 276 nm) of the crude...
example 4
Synthesis of Rapamvcin-3 1-Divinyl Sulfone and Conjugation to a Protein Carrier
[0034] Preparation of a rapamycin-31-divinyl sulfone hapten (Rapa-DVS): Vinyl sulfone (82.6 mg, 0.7 .mu.mol) was added to a mixture of rapamycin (5.0 mg, 5.5 .mu.mol) and dried anhydrous potassium carbonate (30 mg) in 3 mL of dry acetone at room temperature under a. nitrogen atmosphere. The mixture was stirred for 19 hours. Passing a stream of nitrogen through the flask evaporated the solvent and the resulting residue was immediately quenched with 5 mL of a solution of 10 drops of acetic acid in 10 mL methanol. The clear solution was then decanted off the potassium carbonate granules and the solution concentrated. The residue was passed through a silica gel column using a gradient of methanol / chloroform (1% to 2% methanol) as eluent to separate the reaction products from excess vinyl sulfone. The combined reaction products were then purified and analyzed as follows.
[0035] Analysis of a rapamycin-31-diviny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com