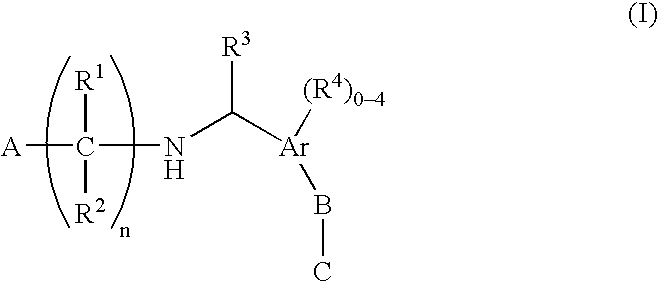
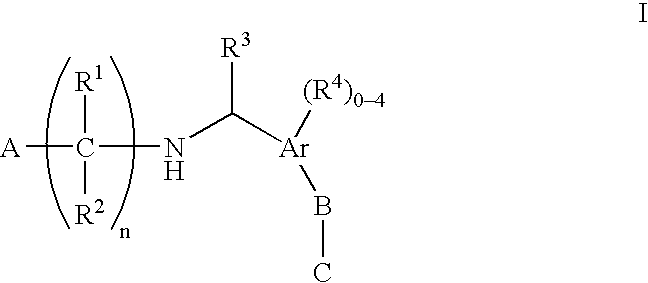
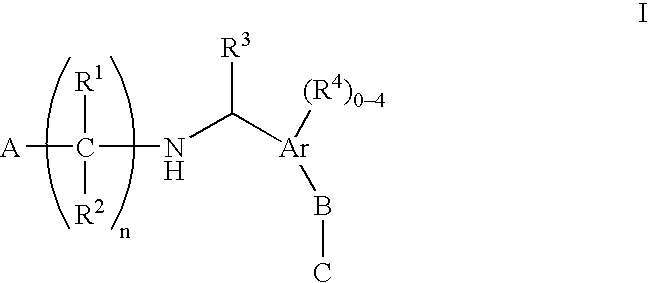
N-(benzyl)aminoalkylcarboxylates, phosphinates, phosphonates and tetrazoles as edg receptor agonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-((4-Decyloxy)benzyl)-3-aminopropylphosphonic acid
3-Aminopropylphosphonic acid (0.064 g, 0.457 mmol) and tetrabutylammonium hydroxide (1.0M in methanol, 0.46 mL, 0.46 mmol) in methanol (3 mL) were heated at 50° C. for 1 h to dissolve all solids. 4-(Decyloxy)benzaldehyde (0.100 g, 0.381 mmol) and sodium cyanoborohydride (0.025 g, 0.40 mmol) were added and stirring was continued for 1 h at 50° C. The reaction mixture was made acidic (pH˜5) by the addition of concentrated HCl then directly purified by LC-3 to give the title compound (0.055 g): 1H NMR (500 MHz , CD3OD) δ 7.39 (d, J=8.7 Hz, 2H), 6.98 (d, J=8.7 Hz, 2H), 4.12 (s, 2H), 3.99 (t, J=6.4 Hz, 2H), 3.12 (t, J=7.7 Hz, 2H), 2.0 (m, 2H), 1.64-1.84 (m, 4H), 1.47 (m, 2H1), 1.24-1.40 (m, 12H), 0.90 (t, J=6.9 Hz, 3H); MS m / e 386.4 (M+H).
examples 2-107
The following Examples (2-112) were prepared using a procedure analogous to that described in EXAMPLE 1 substituting A for 4-(decyloxy)benzaldehyde and B for 3-aminopropylphosphonic acid.
EXAMPLEABESI-MS2358.21H NMR (500 MHz, CD3OD) δ 7.35-7.41 (m, 2H), 6.94-7.01 (m, 2H), 4.08-4.13 (m,2H), 3.96-4.02 (m, 2H), 3.08-3.14 (m, 2H), 1.93-2.04 (m, 2H), 1.73-1.82 (m, 4H),1.43-1.51 (m, 2H), 1.26-1.41 (m, 8H), 0.87-0.94 (m, 3H).3372.21H NMR (500 MHz, CD3OD) δ 7.38 (d, 2H), 6.98 (d, 2H), 4.86 (s, 19H), 4.12 (s,2H), 3.98 (t, 2H), 3.12 (t, 2H), 1.94-2.04 (m, 2H), 1.72-1.84 (m, 4H), 1.42-1.52 (m,2H), 1.24-1.41 (m, 8H), 0.90 (t, 3H).4400.21H NMR (500 MHz, CD3OD) δ 7.36-7.40 (m, 2H), 6.95-7.01 (m, 2H), 4.12 (s, 2H),3.95-4.02 (m, 2H), 3.09-3.15 (m, 2H), 1.94-2.04 (m, 2H), 1.72-1.84 (m, 4H), 1.42-1.52(m, 2H), 1.24-1.42 (m, 8H), 0.87-0.94 (m, 3H).5336.21H NMR (500 MHz, CD3OD) δ 7.33-7.44 (m, 5H), 7.27-7.33 (m, 2H), 7.03-7.09 (m,2H), 5.11 (s, 2H), 4.11 (s, 2H), 3.07-3.15 (m, 2H), 1.92-2.04 (m, 2H), 1...
example 108
(R / S)-3-(N-(4-Nonylbenzyl)amino-1-hydroxypropylphosphonic acid
Step A: (R / S)-Diethyl 3-benzyloxycarbonylamino-1-hydroxypropylphosphonate
To a solution of potassium bis(trimethylsilyl)amide (1.13g, 5.66 mmol) in tetrahydrofuran (10 mL) at 0° C. was added diethyl phosphite (0.73 g, 5.66 mmol). After 10 min, 3-(benzyloxycarbonylamino)propanal (0.78 g, 3.77 mmol) was added as a solution in tetrahydrofuran (5 mL). After 30 min, the reaction was quenched by the addition of 2N hydrochloric acid (25 mL) and extracted with ethyl acetate (50 mL). The organic layer was washed with sat'd sodium chloride (50 mL), dried over magnesium sulfate and concentrated in vacuo. Silica gel chromatography eluting with hexane / acetone (1:1) gave a colorless oil (0.36 g): ESI-MS 346.1 (M+H).
Step B: (R / S)-Diethyl 3-amino-1-hydroxypropylphosphonate
(R / S)-Diethyl 3-benzyloxycarbonylamino-1-hydroxypropylphosphonate (0.36 g, 1.04 mmol, from Step A) and palladium on carbon (10%, 0.10 g) were stirred together in m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com