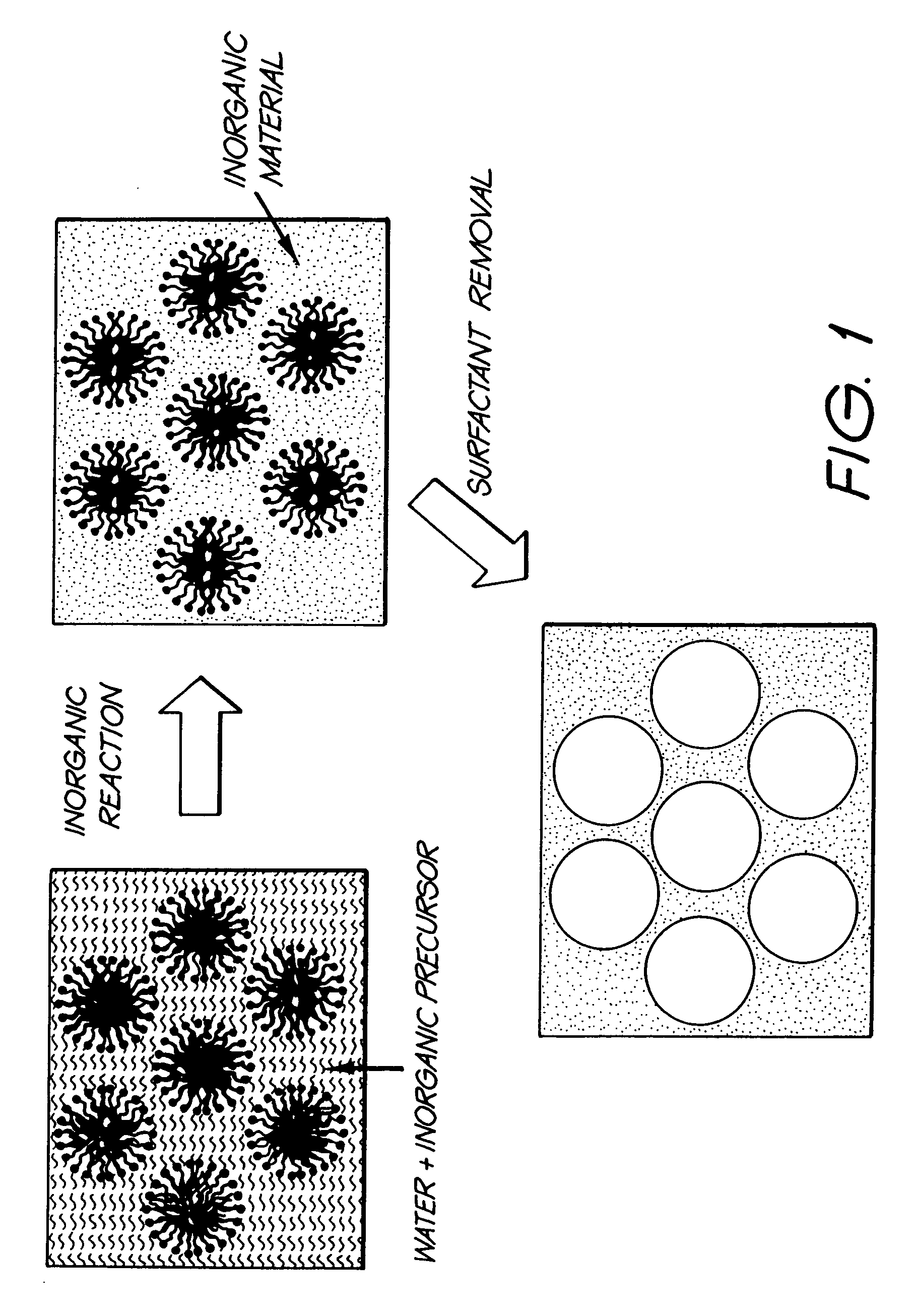
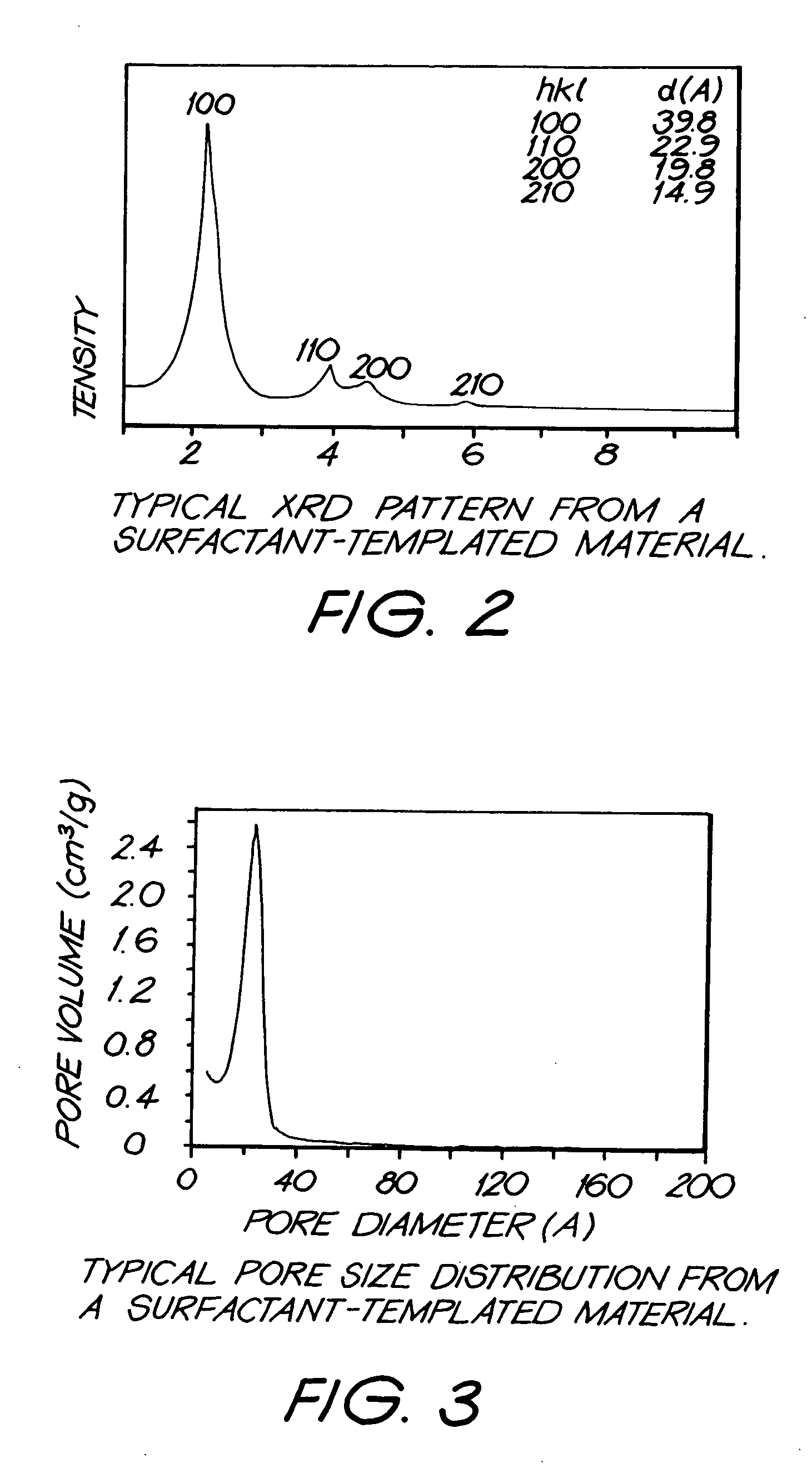
Production of fine-grained particles
a technology of fine grains and metal oxides, applied in the direction of nickel compounds, lanthanide oxides/hydroxides, cobalt carbonyls, etc., can solve the problems of difficult uniform dispersion of different elements at the ultra-fine scale required for nanometre-sized grains, and the reported process used to achieve fine grain size is very expensive, so as to achieve a wide distribution of pore sizes and large specific surface areas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of CeO2
[0107] In order to demonstrate the method of the present invention, particles of CeO2 were produced. The following procedure was used:
[0108] Step 1: A cerium nitrate solution containing 2.5 moles / litre cerium nitrate was prepared.
[0109] Step 2: 16 g Brij 56 surfactant and 20 mls cerium nitrate solution were heated to ˜80° C. At this temperature the surfactant is a liquid. The solution was added slowly to the surfactant liquid while stirring, to create a micellar liquid.
[0110] Step 3: The micellar liquid was cooled to room temperature. During the cooling the liquid transformed to a clear gel.
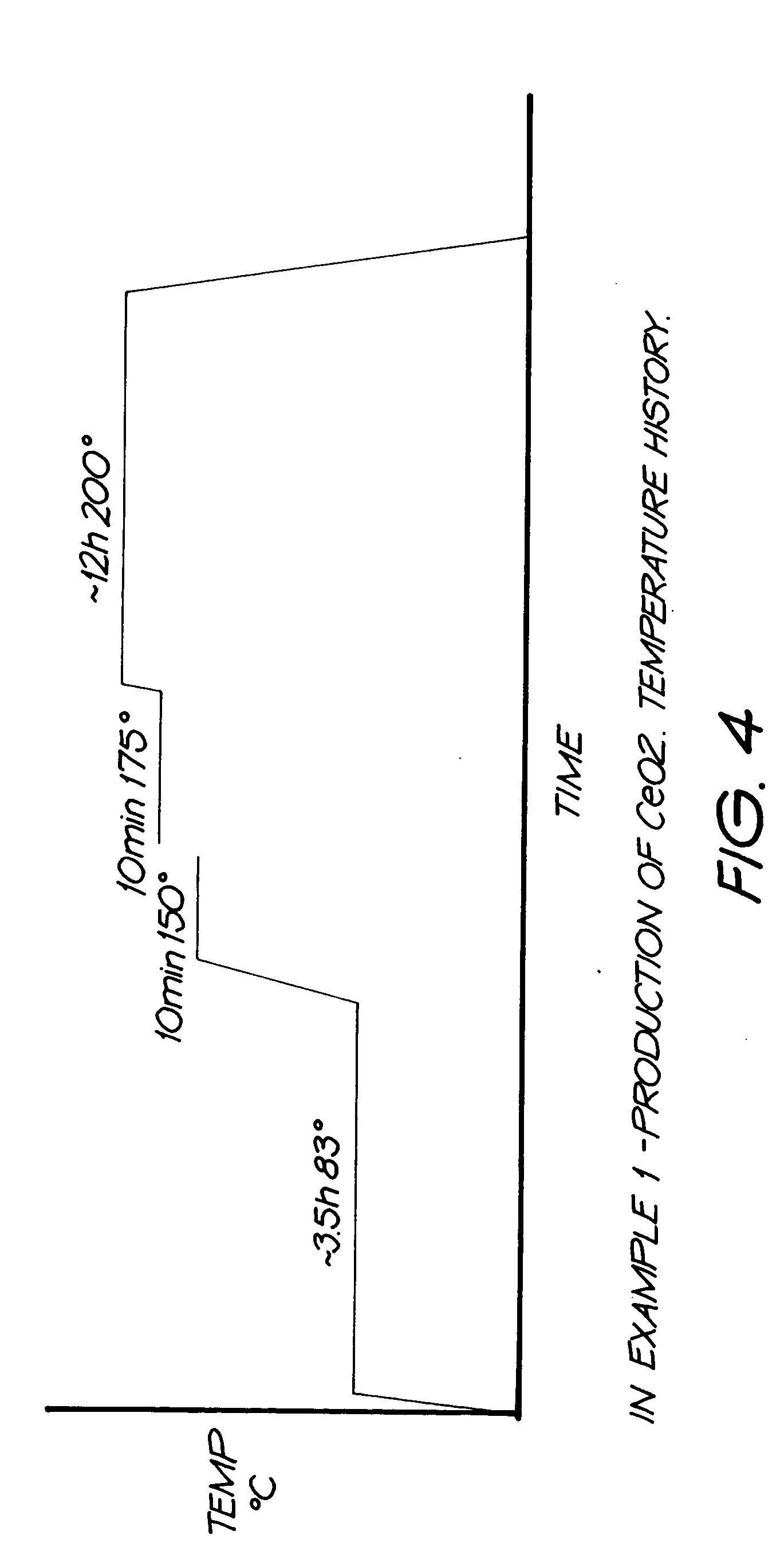
[0111] Step 4: The gel was heat treated according to temperature history presented in FIG. 4. In this example, an extended drying stage at 83° C. was used prior to further heating.
[0112] The resulting CeO2 powder had a surface area of ˜253m2 / g, and was comprised of grains that ranged between ˜2 and ˜8 nm in diameter. Transmission electron microscopy (TEM) suggests that the...
example
Preparation of La0.6Ca0.2Nd0.2Mn0.9Ni0.1O3
[0122] La0.6Ca0.2Nd0.2Mn0.9Ni0.1O3 is used as the cathode material in solid oxide fuel cells. It is also an excellent test material for the present invention because the target ‘lanthanum manganate’ crystal structure is extremely sensitive to chemical composition. Even small variations in composition result in the formation of different crystal structures. Therefore, the five different metal elements need to be evenly distributed on an extremely fine scale to produce small grains with the correct crystal structure.
[0123] Using co-precipitation and other conventional processes, previous researchers have had considerable difficulty in obtaining the correct crystal structure because of this sensitivity to composition. Careful co-precipitation, followed by long (10h-48h) heat treatments at high temperatures (800-1000° C.) have been necessary to attain the correct crystal structure in the prior art (variations in chemical composition can be all...
PUM
| Property | Measurement | Unit |
|---|---|---|
| grain size | aaaaa | aaaaa |
| grain size | aaaaa | aaaaa |
| grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com