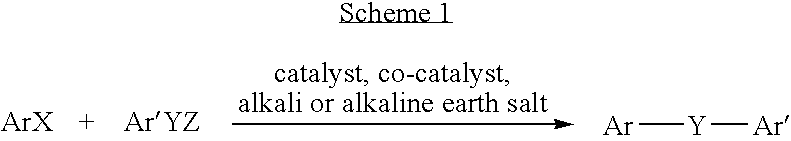
Diaryl ether condensation reactions
a technology of diaryl ether and condensation reaction, which is applied in the preparation of ether, ether/acetal/ketal group formation/introduction, carboxylic compound preparation, etc., can solve the problems of high yield of reaction, and low yield of reaction, etc., to achieve the effect of high yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
REFERENCES AND FOOTNOTES FOR EXAMPLE 1
(1) Pellón, R. F.; Carrasco, R.; Milián, V.; Rodes, L. Synth. Commun. 1995, 25, 1077 and references cited therein. (2) For recent reviews on natural biphenyl ether peptides, see: (a) Evans, D. A.; DeVries, K. M. In Glycopeptide Antibiotics, Drugs and the Pharmaceutical Sciences; Nagarajan, R., Ed.; Marcel Decker, Inc.: New York, 1994; Vol. 63, pp. 63-104. (b) Itokama, H.; Takeya, K. Heterocycles 1993,35, 1467. (3) Kase, H.; Masami, K.; Yamada, K. J. Antibiot. 1987, 40, 450. (4) (a) Sano, S.; Ikai, K.; Kuroda, H.; Nakamura, T.; Obayashi, A.; Ezure, Y.; Enomoto, H. J. Antibiot. 1986, 39, 1674. (b) Sano, S.; Ikai, K.; Katayama, K.; Takesako, K.; Nakamura, T.; Obayashi, A.; Ezure, Y.; Enomoto, H. J. Antibiot. 1986, 39, 1685. (5) Jolad, S. D.; Hoffmann, J. J.; Torrance, S. J.; Wiedhopf, R. M.; Cole, J. R.; Arora, S. K.; Bates, R. B.; Gargiulo, R. L.; Kriek, G. R. J. Am. Chem. Soc. 1977, 99, 8040. (6) (a) Itokawa, H.; Takeya, K.; Mihara, K.; Nob...
example 2
REFERENCES FOR EXAMPLE 2
(1) Pellon, R. F.; Carrasco, R.; Millian, V.; Rodes, L. Synth. Commun. 1995, 25, 1077-1083 and references cited therein. (2) For a review, see Evans, D. A.; DeVries, K. M. In Glycopeptide Antibiotics, Drugs and the Pharmaceutical Sciences; Nagarijan, R., Ed.: Marcel Decker, Inc.: New York, 1994 Vol 63, pp-63-104. (3) J. Antibiot. 1987, 40, 450454 (4) J. Antibiot. 1986, 39, 1674-1684; J. Antibiot. 1986, 39, 1685-1696; J. Antibiot. 1986, 39, 1696-1703. (5) J. Am. Chem. Soc. 1983, 105, 1343; J. Am. Chem. Soc. 1977, 99, 8040. (6) Chem. Pharm. Bull. 1984,32, 284; Chem. Pharm. Bull 1983, 31, 1424. (7) William, D. H. Acc. Chem. Res. 1984, 17, 364-369. (8) Janetka, J. W.; Ramana, P.; Satyshur, K.; Flentke, G. R.; rich, D. H. J. Am. Chem Soc. 1997, 119,441-442. (9) Ullmann, F. Ber. 1904, 37, 853. (10) Lindley, J. Tetrahedron 1984, 40, 1433-1456.
example 3
Copper-Catalyzed N-Arylation of Amides
The use of catalytic quantities of dibenzylidene acetone (dba) and 1,10-phenanthroline (phen) promote the copper catalyzed N-arylation of various amides with aryl bromides.
The yields for various permutations of the reaction conditions, as assessed by gas chromatography, are as follows:
Amide (R)without dba + phenwith phenwith dba + phenH<5—89CH3<51597 (83% isolated)C6H50—67
2,2′-Bipyridine was also found to be an effective ligand for this arylation, although it was not as effective as 1,10-phenanthroline.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| Lewis basic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com