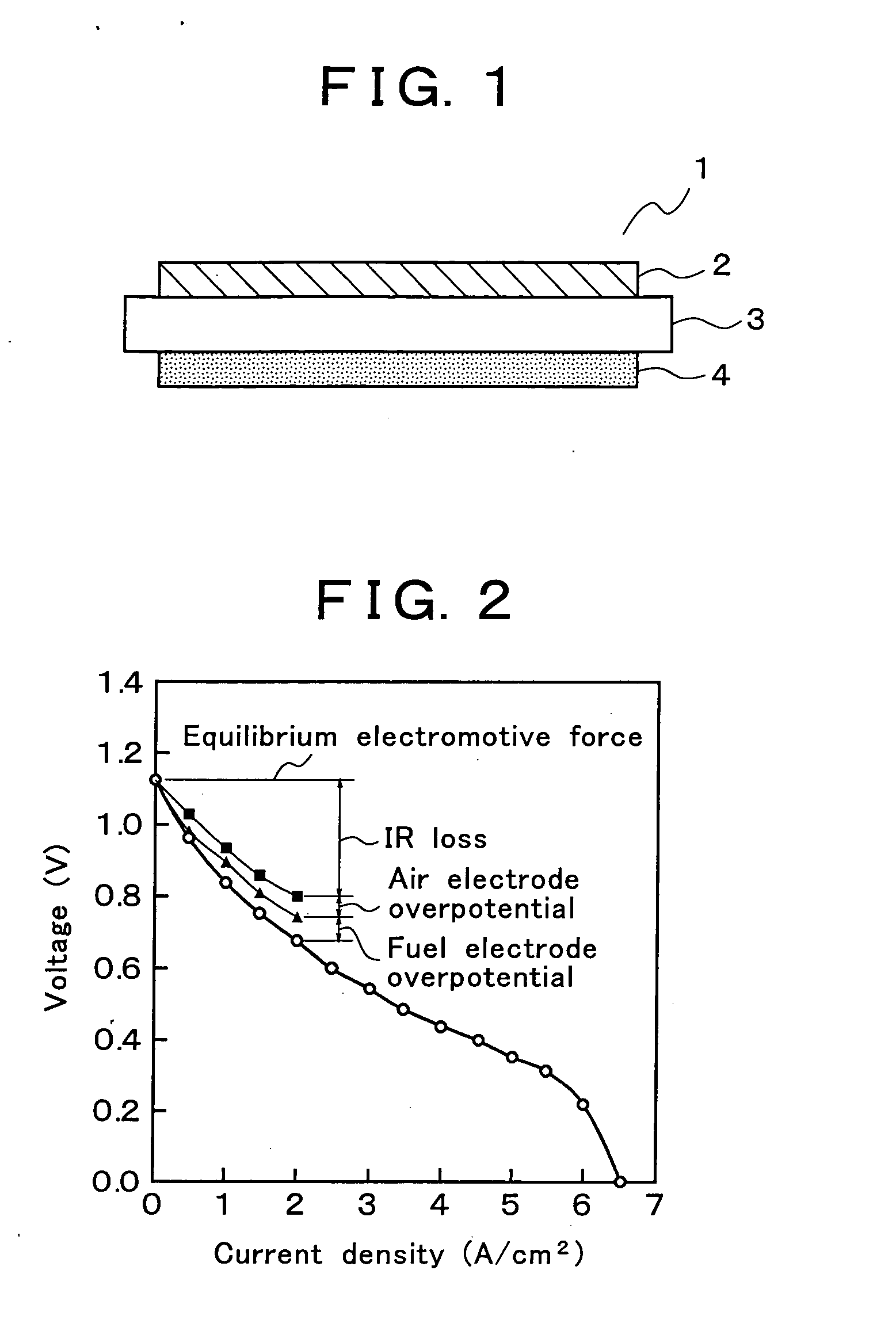
Solid oxide fuel cell and manufacturing method thereof
a solid oxide fuel cell and manufacturing method technology, applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of ir loss, voltage drop with increasing irreversibility, etc., to reduce the overpotential of each electrode and ir loss, and improve the efficiency of electric power generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0032] Step 1: Fabrication of a Solid Electrolyte Layer
[0033] As source materials, La2O3, SrCO3, Ga2O3, MgO, and CoO were prepared, weighed out in predetermined amounts, subjected to mixing with the aid of a ball mill, heated in air at 1,200° C. for calcination, and additionally pulverized with the aid of a ball mill, and thus a lanthanum gallate based oxide powder was produced. The lanthanum gallate based oxide powder thus obtained was molded into a thin plate shape with the aid of a method well known in the art such as the doctor blade method and the like, and then heated at 1,450° C. in air; thus the solid electrolyte material plates (Examples 1 to 4) of the compositions and thickness values shown in Table 1 were manufactured.
[0034] Step 2: Production of the Fuel Electrode Layer
[0035] In each of the present Examples, the fuel electrode layer was produced by the spray thermal decomposition method described below.
[0036] Nickel nitrate, cerium nitrate and samarium nitrate were p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average primary grain size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com