Hybridization and mismatch discrimination using oligonucleotides conjugated to minor groove binders
a technology of oligonucleotide and minor groove binder, applied in the field of molecular biology, can solve the problems of limiting restricting the use of longer oligonucleotides, and limiting its versatility, so as to increase the degree of hybridization, increase the stability of hybrids, and increase the stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
MGB-Oligonucleotide Conjugates as PCR Primers
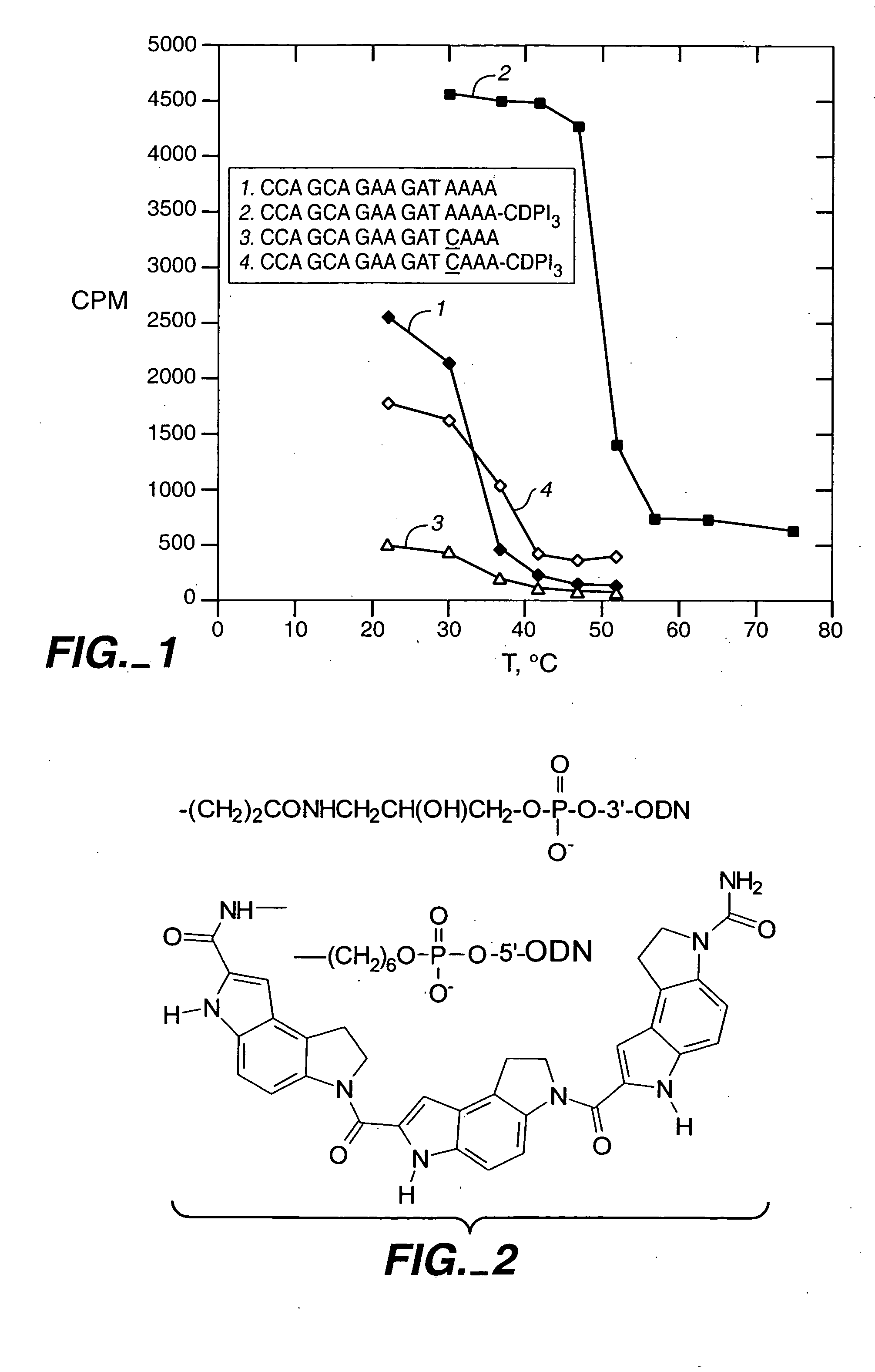
In this example, we show that a modification which greatly improves hybrid stability of a short oligonucleotide also allows the oligonucleotide to serve as a PCR primer. CDPI3; the trimer of 1,2-dihydro-(3H)-pyrrolo[3,2-e]indole-7-carboxylate, or CDPI; is a synthetic non-reactive derivative of a subunit of the antitumor antibiotic CC-1065 (Hurley et al. (1984) Science 226:843-844). This oligopeptide is a DNA minor groove binder (MGB), with a very high affinity for the minor groove of A-T-rich double-stranded DNA. It has previously been reported that, when compared to unmodified oligonucleotides of the same length, CDPI3-oligonucleotide conjugates form unusually stable and specific hybrids with complementary single-stranded DNA (Lukhtanov et al (1995) Bioconjugate Chem. 6:418-426; Afonina et al. (1996) Proc. Natl. Acad. Sci. USA 93:3199-3204). This example demonstrates that conjugates of short oligonucleotides with CDPI3 make effective p...
example 2
Use of MGB-Oligonucleotide Conjugates in a Hydrolyzable Probe Assay
In this example, we show that conjugation of MGBs to short oligonucleotides results in improved hybrid stability and improved discrimination between a perfect hybrid and a single-base mismatch, when MGB-short oligonucleotide conjugates are used in a hydrolyzable probe assay. The procedure described by Wittwer et al. (1997a) BioTechniques 22:130-138, was used. In this method, MGB-oligonucleotide conjugates, additionally comprising a fluorophore and a quenching agent, were used as probes in a hydrolyzable probe assay. This type of probe is designed to be complementary to a predicted amplification product, and emits very little or no fluorescence, due to the proximity of the fluorophore to the quenching agent. Formation of a hybrid between the probe and the amplification product produces a structure that is a substrate for exonucleolytic hydrolysis of the probe by a polymerase possessing duplex-specific exonuclease ac...
example 3
Effect of Nucleotide Analogues on Hybridization Strength and Discriminatory Ability of MGB-Oligonucleotide Conjugates
Further increases in discriminatory ability of a MGB-oligonucleotide conjugate are obtained when the conjugate also contains a pyrazolo[3,4-d]pyrimidine nucleotide analogue. In this system, the target sequence is located in the E. coli supF gene contained in the plasmid pSP189 (FIG. 5, SEQ ID No.: 40). See Parris et al. (1992) Gene 117:1-5. Binding sites for the primers used for amplification are indicated as Primer 1 and Primer 2, with Primer 1 having a sequence and polarity that is identical to that shown in FIG. 5, and Primer 2 having a sequence and polarity that is the reverse complement to that shown in FIG. 5. A 15-mer probe, labeled with fluorescein at the 5′-end, and with TAMRA and CDPI3 at the 3′-end, was designed to be complementary to a region within the approximately 375 nucleotides between the primers, as indicated in FIG. 5. This probe was tested using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com