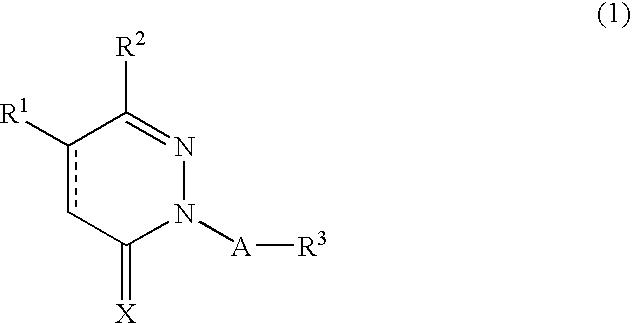
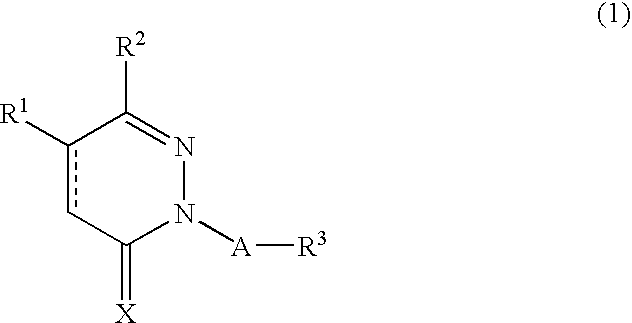
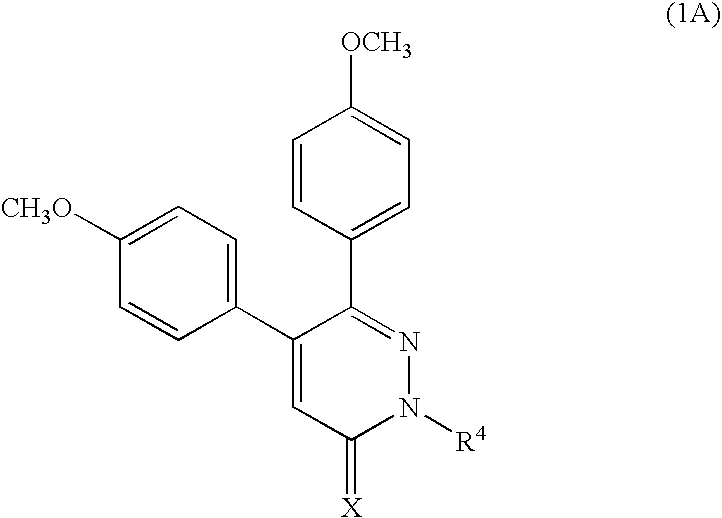
Novel pyridazine derivatives and medicines containing the same as effective ingredients
a technology of pyridazine and derivatives, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problem that none of these compounds can exhibit sufficient inhibitory activity against interleukin-1 production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
(1) Preparation of 3,4-bis(4-methoxyphenyl)-2-hydroxy-4-oxobutanoic Acid
To a solution of sodium periodate (11.1 g, 52.0 mmol) in water (65 ml), concentrated sulfuric acid (1.12 ml) was added dropwise little by little under ice-water cooling and stirring. Subsequent to the dropwise addition, the temperature of the resulting mixture was allowed to rise to room temperature, followed by the addition of a solution of tartaric acid (7.81 g, 52.0 mmol) in water (18 ml). The mixture was stirred for 50 minutes. To the reaction mixture, an aqueous solution of sodium hydroxide and a suspension of 2-(4-methoxyphenyl)-4′-methoxyacetophenone (13.32 g, 52.0 mmol) in ethanol (160 ml) were added. The mixture was stirred at 40° C. for 5 hours and then at room temperature for 17 hours, and a reaction was then conducted at 70° C. for 1 hour. Subsequent to cooling, the ethanol was distilled off. The liquid residue was washed with ethyl acetate, acidified with hydrochloric acid, and then extracted with...
preparation example 2
Preparation of Methyl 4-(4-methoxyphenyl)-4-oxo-3-(4-pyridyl)butanoate
Under an argon, 2-(4-pyridyl)-4′-methoxyacetophenone (J. Am. Chem. Soc., 112, 2163-3168, 1990: Dimitrios Stefanidis and John W. Bunting; 9.6 g, 42.3 mmol) was suspended in tetrahydrofuran (200 ml), and under ice cooling, lithium diisopropylamide (2.0 M solution; 25 ml, 50.0 mmol) was added. At the same temperature, the mixture was stirred for 30 minutes. Methyl bromoacetate (6.0 ml, 63.4 mmol) was then added dropwise, and the mixture was stirred under ice cooling for 1 hour and then at room temperature for 2 hours. The reaction mixture was diluted with toluene. The mixture was washed successively with 2 N hydrochloric acid, water and a brine, and was then dried over anhydrous sodium sulfate. The solvent was distilled off and the residue was separated and purified by chromatography on a silica gel column [silica gel: 100 g, hexane / ethyl acetate (1 / 2)], whereby the title compound (10.63 g, 84.1%) was obtained as a...
preparation example 3
(1) Preparation of 2-(4-chlorophenyl)-4′-(methylthio)acetophenone
A mixture consisting of para-chlorophenyl acetic acid (17.06 g, 0.1 mol), thioanisole (24.84 g, 0.2 mol) and polyphosphoric acid (67.59 g, 0.2 mol) was heated at 100° C. for 7 hours. Water was added to the solidified reaction product, and a white solid insoluble in water was collected by filtration and then washed with n-hexane. The solid was recrystallized from a mixed solvent of ethanol and ethyl acetate, whereby the title compound (21.24 g, 76.7%) was obtained. Further, the mother liquor was separated and purified by chromatography on a silica gel column (ethyl acetate). Recrystallization was then conducted from ethyl acetate, whereby the title compound (2.86 g, 10.4%) was obtained.
Colorless prisms (ethyl acetate)
Melting point: 161.1-162.1° C.
1H-NMR (CDCl3) δ: 2.51(3H,s), 4.21(2H,s), 7.19(2H,d,J=8.55 Hz), 7.26(2H,d,J=8.91 Hz), 7.29(2H,d,J=8.55 Hz), 7.89(2H,d,J=8.91 Hz).
(2) Preparation of Ethyl 3-(4-chlorophe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com