Electrophoretic in situ tissue staining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
[0036] Electrophoretic Tissue Staining using Anti-CD34 Antibody in Tonsil.
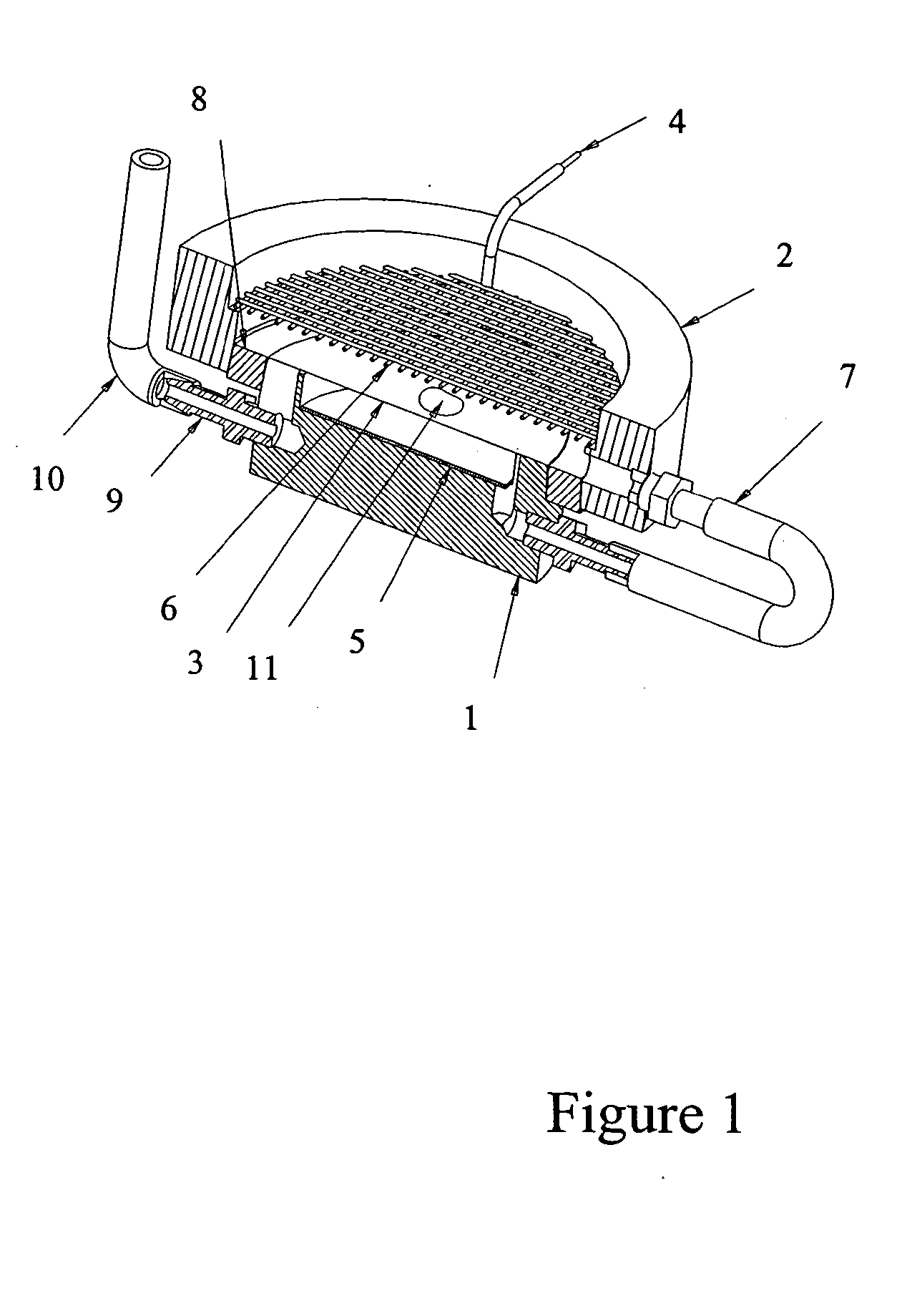
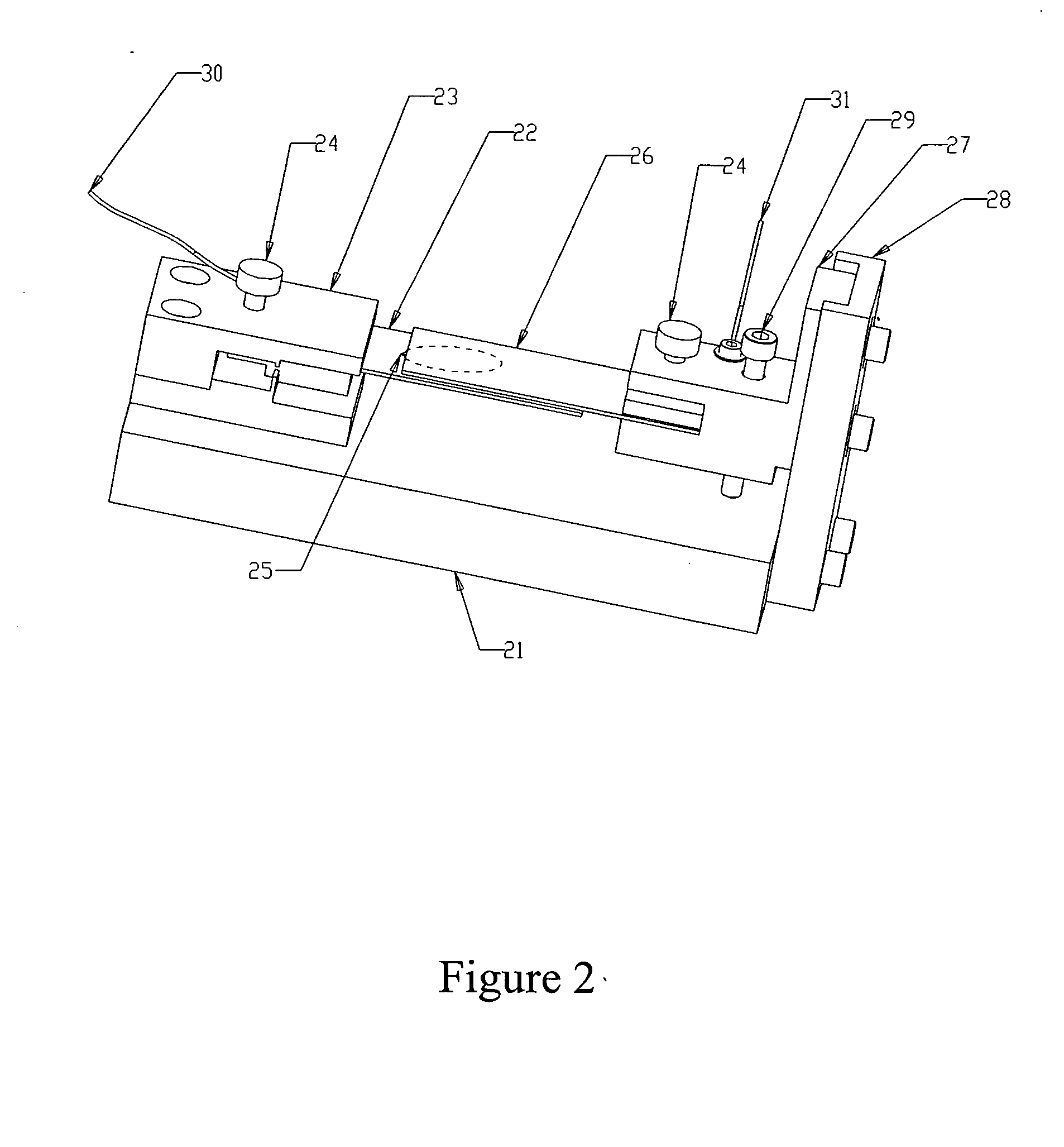
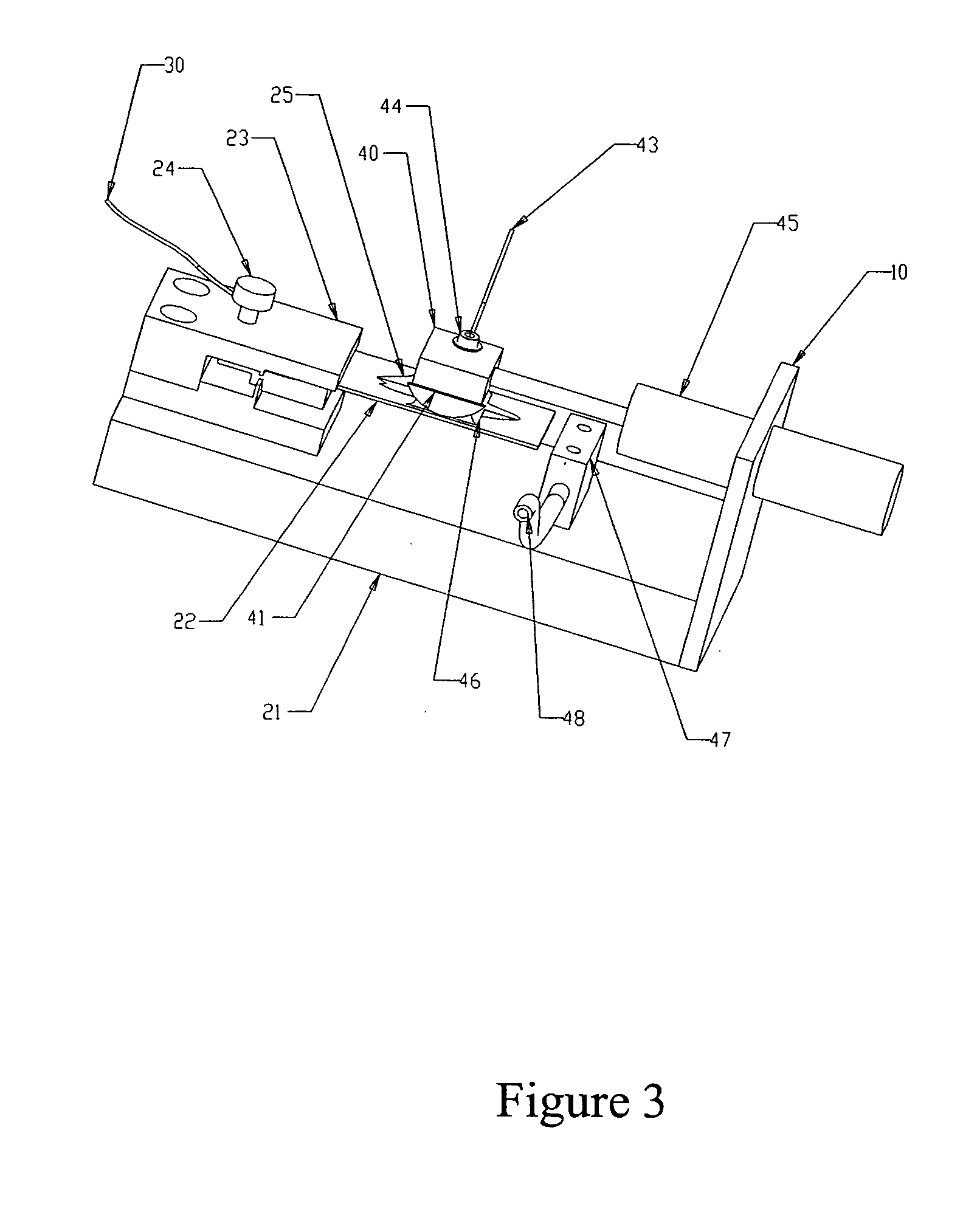
[0037] The following experiment was run to determine if antibody could be introduced electrophoretically into tissue. The tissue was adhered to a hydrophilic polytetrafluoroethylene (PTFE) membrane (TEFLON® Plumber's Tape) to enable manipulation and orientation of the tissue in the gel, and then embedded in an agarose gel for subsequent electrophoresis.
[0038] Procedure: four sections of 5 μm-thick human tonsil were mounted to PVA-treated hydrophilic PTFE membrane, air dried for 48 hours, overnight dried at 60° C., manually de-paraffinized and re-hydrated (standard process of dipping sections sequentially in xylene, then 100% EtOH, 90% EtOH, 80% EtOH, 70% EtOH, and finally 100% H2O). The PTFE membrane was made hydrophilic by wetting in Isopropyl alcohol first, then soaking for several hours in a solution of 0.1% polyvinyl alcohol in phosphate buffer, pH 2.2 and 5% glutaraldehyde, and rinsed in DI water. Any h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric field | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com