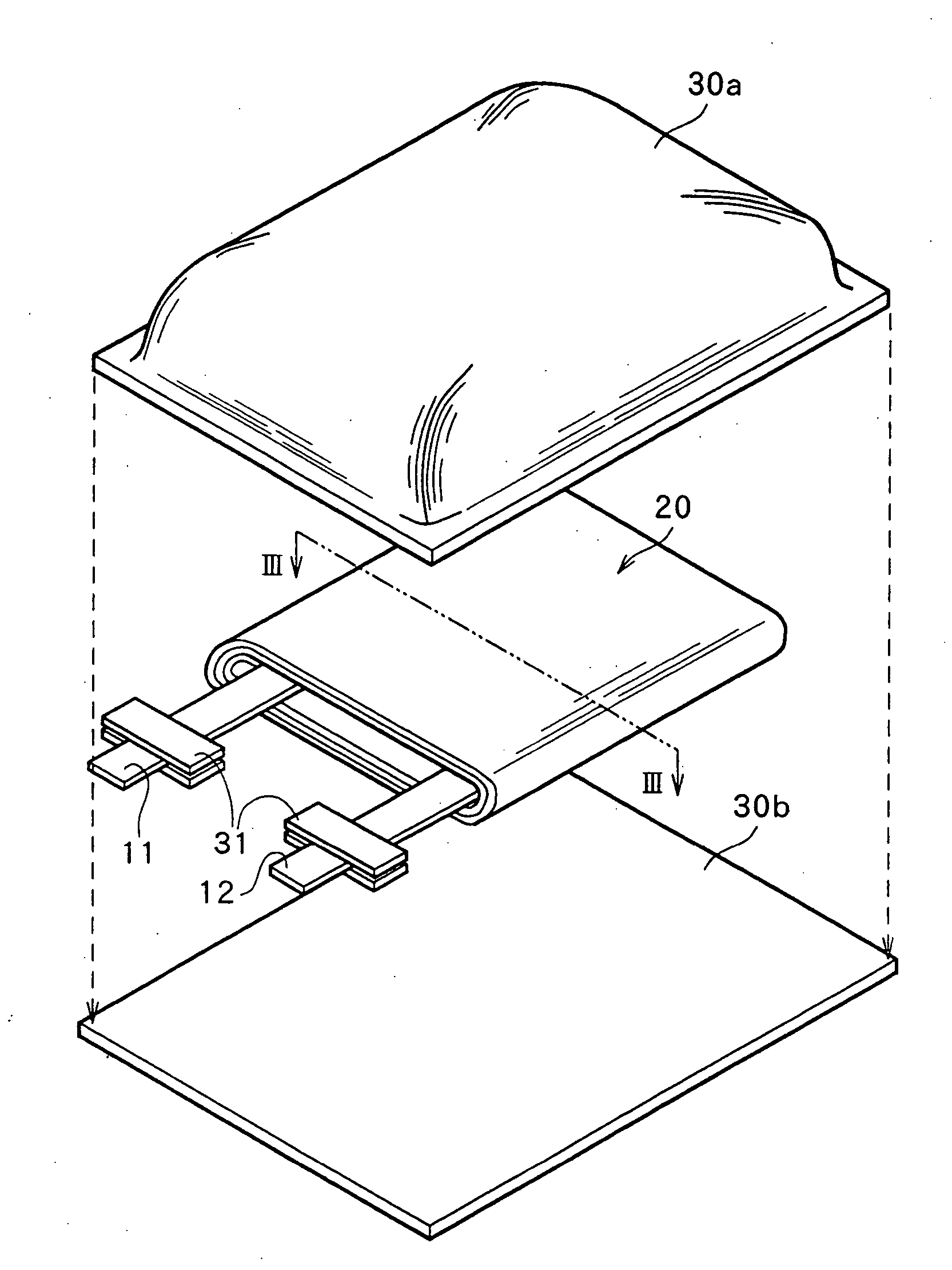
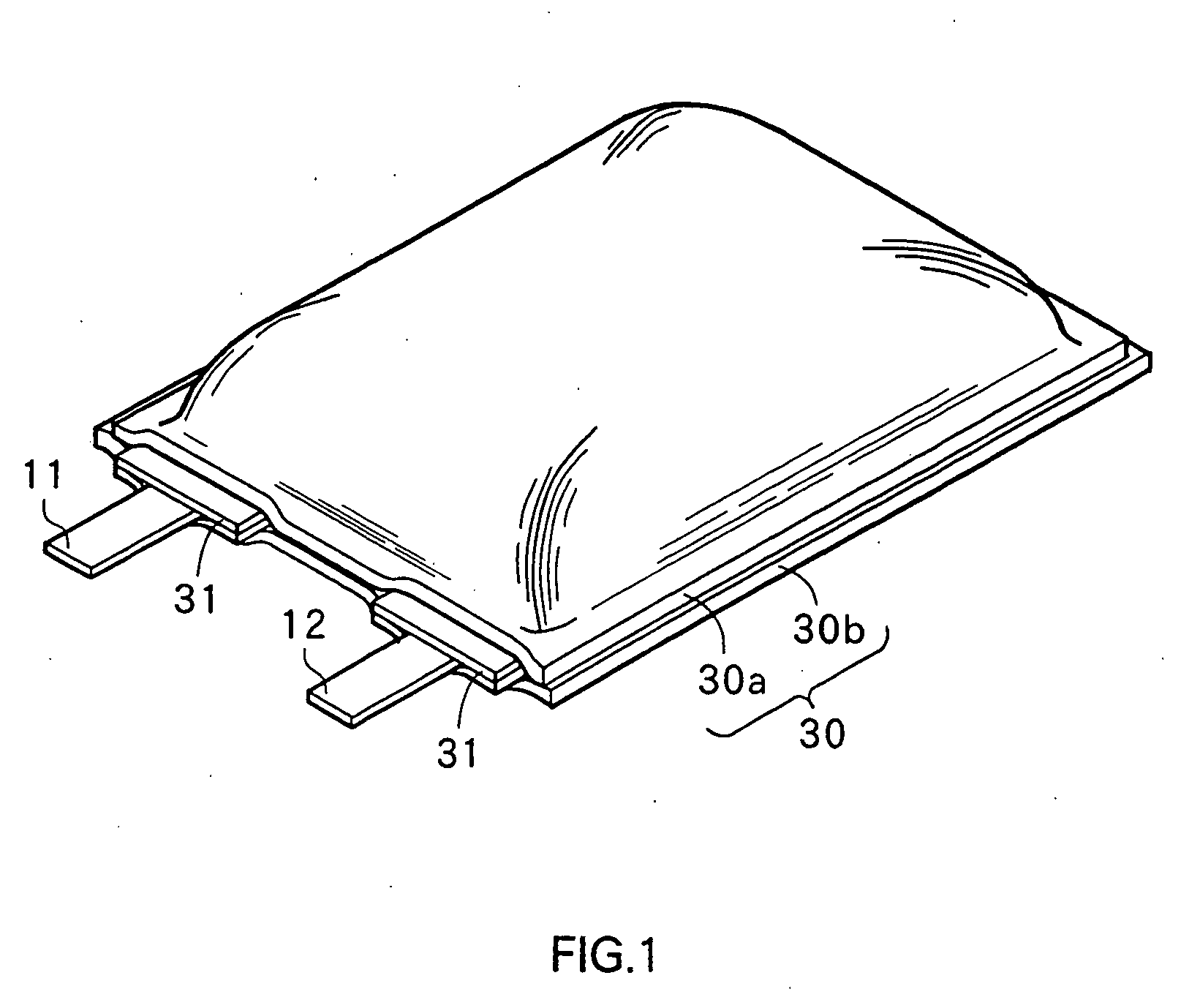
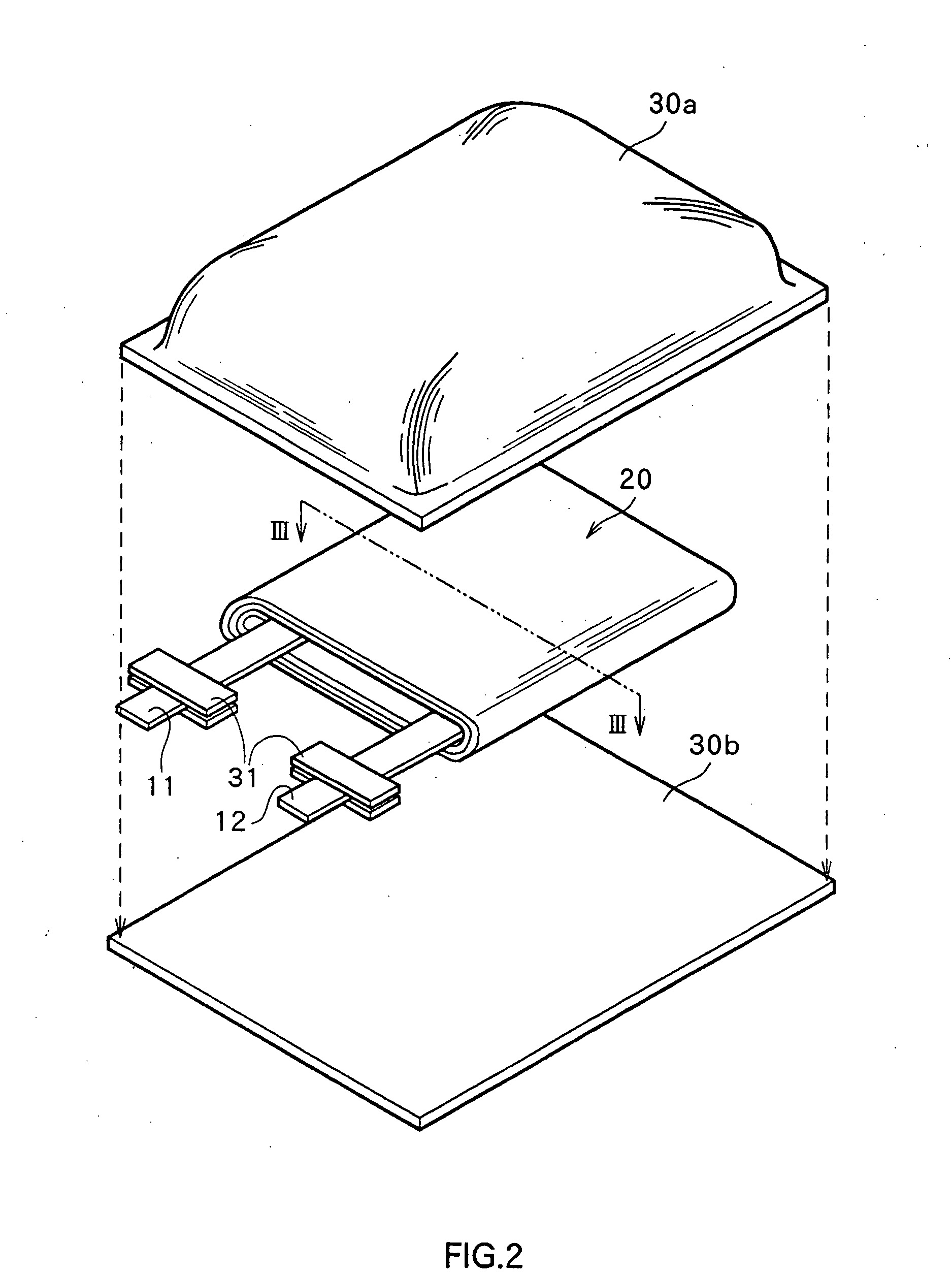
Non-aqueous electrolyte secondary battery
a secondary battery, non-aqueous electrolyte technology, applied in the field of batteries, can solve the problems of strong recent demands for a lighter, thinner, smaller secondary battery, and insufficiently addressed demand regarding shape, and achieve the effect of suppressing shape change and suppressing deterioration in battery characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0069] Further, examples of the invention will be described in detail.
examples 1-1 to 1-31
[0070] First, a copolymer of vinylidene fluoride and hexafluoropropylene as polymer materials was dissolved in a solvent obtained by mixing propylene carbonate and ethylene carbonate, and further, LiPF6 was dissolved as a lithium salt. The mixing ratio in volume of the solvent and the polymeter material, specifically, propylene carbonate:ethylene carbonate:copolymer was set to 4:4:1. LiPF6 was dissolved at the rate of 0.74 mol / dm3.
[0071] The mixture solution was stored in a drying chamber for one week or longer and heated to about 70° C. so as to be gelled. In such a manner, electrolytes of the Examples 1-1 to 1-31 were obtained. The electrolytes of the Examples 1-1 to 1-31 were fabricated separately under the same conditions.
[0072] The concentration of the free acid (hydrogen fluoride in this case) of the obtained electrolyte was measured. To be specific, the electrolyte is dissolved in cold water of 1.5° C. or lower so as not to be hydrolyzed. After adding bromothymol blue as an...
examples 2-1 to 2-3
[0082] As Examples 2-1 to 2-3, secondary batteries were fabricated in a manner similar to Examples 1-1 to 1-31 except that the concentration in mass ratio of the free acid in the electrolyte was changed as shown in Table 1. As Comparative Examples 2-1 to 2-3 of Examples 2-1 to 2-3, secondary batteries were fabricated in a manner similar to the examples except that the concentration of the free acid in the electrolyte as shown in Table 1.
TABLE 1concentrationinitialcapacityin mass ratiodischargesustainof free acidcapacityratiochange in(ppm)(mAh)(%)shapeExample 2-12558295hardly occursExample 2-25058495hardly occursExample 2-36057193hardly occursComparative10051189expandedExample 2-1Comparative20049484expandedExample 2-2Comparative40048181expandedExample 2-3
[0083] The concentration of the free acid in the electrolyte in each of Examples 2-1 to 2-3 and Comparative Examples 2-1 to 2-3 was controlled by adjusting the drying time in the drying chamber and the gelling temperature. Specific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com