Cosmetic repair using cartilage producing cells and medical implants coated therewith
a cartilage producing cell and cartilage technology, applied in the direction of animal cells, biochemical equipment and processes, skeletal/connective tissue cells, etc., can solve the problems of difficult to accurately control and predict the depth of tissue injury, the use of chemical peels has fallen out of favor, and the risk of hypopigmentation and scarring is increased in the direction of deeper chemical peels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Harvesting Cartilage From a Donor
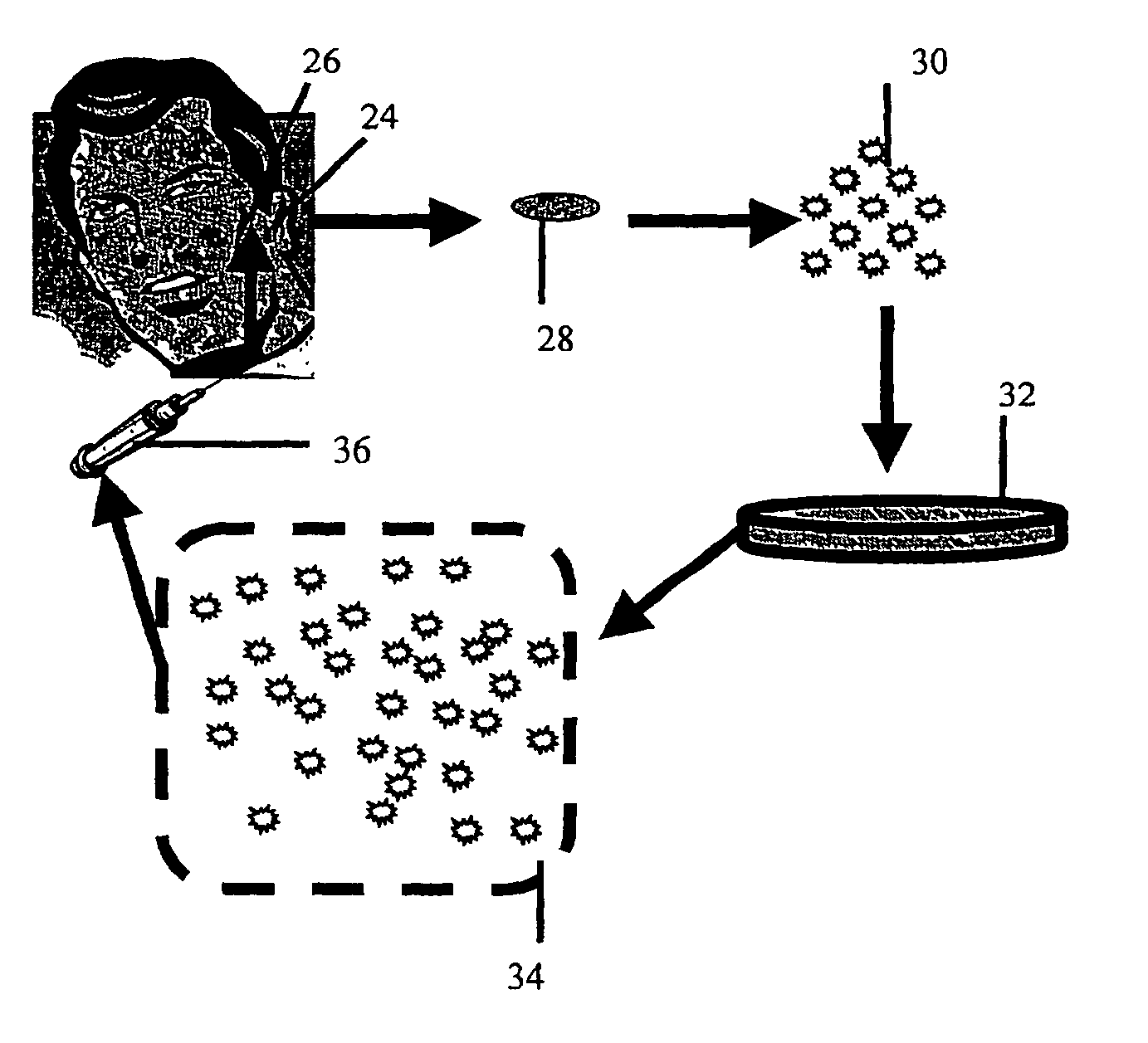
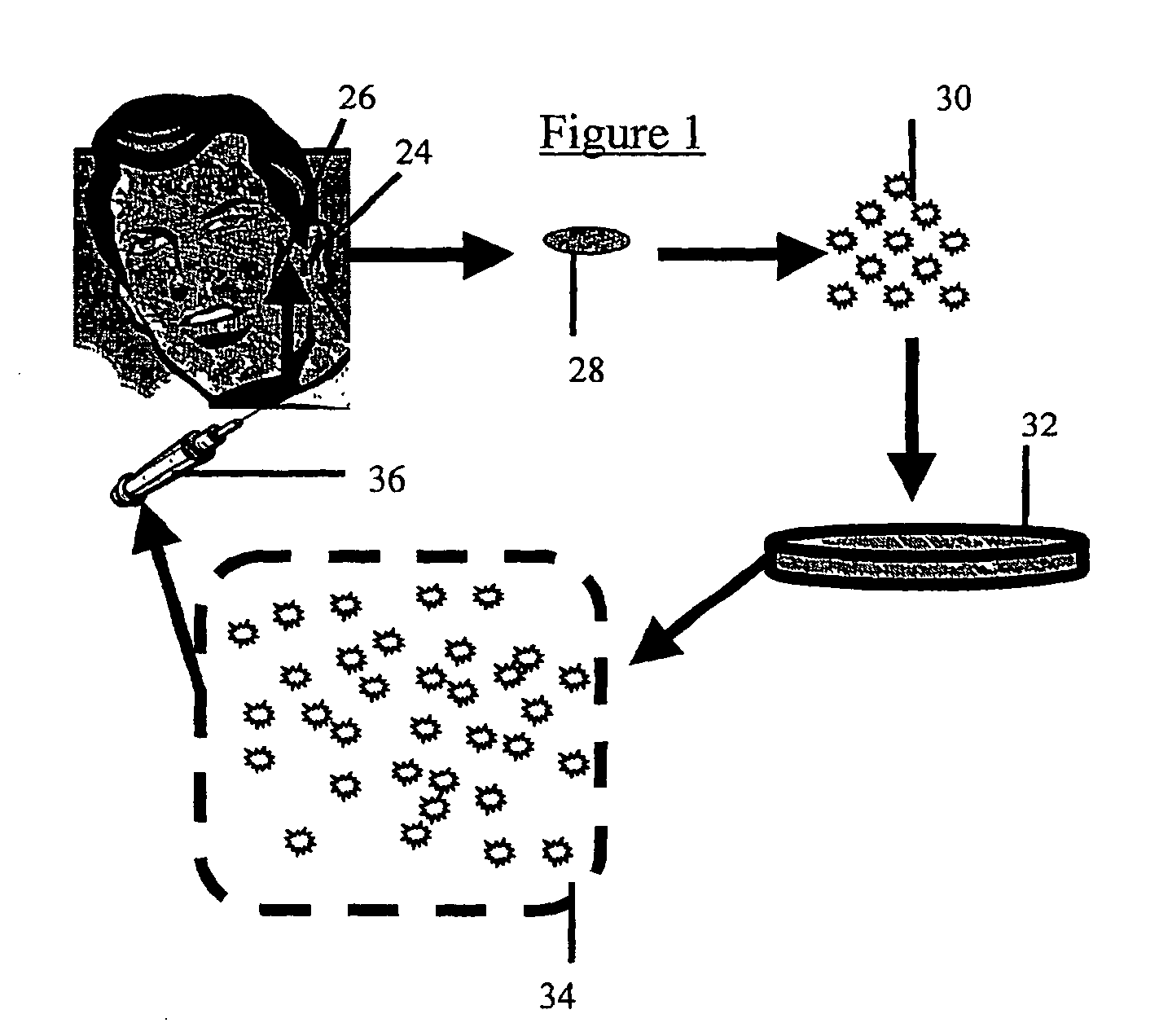
[0063] A cartilage explant of about 1×1 cm. is removed from an incision of approximately 1 cm in length along the posterior side of the concha of the auricle. A number 15 scalpel blade is well suited to this purpose, although other cutting implements may be employed without significantly changing the outcome of the harvest. The incision is then closed with resorbable suture material. The resultant scar is small and hidden by virtue of its location.
[0064] As is further described below, cartilage producing cells enzymatically released from the explant (further described below) can be used directly in cosmetic repair or coating of implants, or alternatively, such cells can be cultured prior to use.
example 2
Culture of Cartilage Producing Cells
[0065] Several methods can be used for harvesting chondrocytes from the cartilage explant and for culturing harvested cells (see for example, Robinson et. al. Autologous chondrocytes transplantation for reconstruction of isolated joint defects: the Assaf Harofeh Experience. Israel Medical Association Journal, Vol. 2:290-295).
[0066] The following describes a method suitable for chondrocyte harvesting and culturing. The cartilage explant is digested with collagenase (0.2% weight / volume) in complete media containing 10% fetal bovine serum, 2 mM L-glutamine, non-essential amino acids, 50 mg / ml proline, 1 mM sodium pyruvate and 35 μg / ml gentamicin for 20 hrs at 37° C. Liberated cells (chondrocytes, chondrocyte progenitors) are spun, resuspended in complete medium, counted and plated at 106 cells per T-150 flask. Cells can be passed at confluence (every 5-7 days) until sufficient cell number are achieved for purposes described in examples 3 and 4.
[00...
example 3
Cosmetic Repair By Introduction of Cartilage Producing Cells
[0068] Chondrocytes produced as describe above are dissociated by brief trypsinization monitored by microscopy. When sufficient disruption of the monolayer has been achieved, cells are washed 2 times in media without fetal calf serum and resuspended in saline or any other physiologically acceptable buffer for injection.
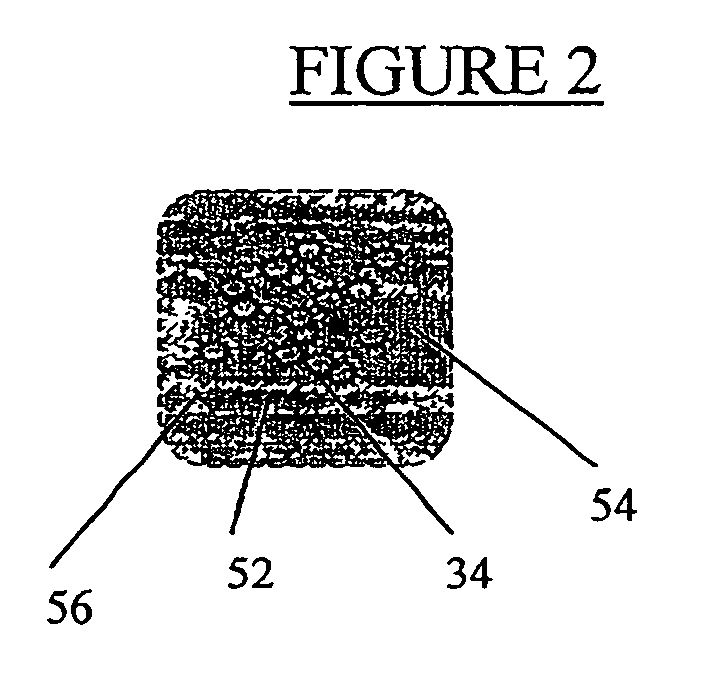
[0069] In order to facilitate injection, a cell density of 106 cells / ml is loaded into a syringe fitted with a needle suitable for subcutaneous injection. Because it is desirable to leave no sign of the injection, a narrow gauge needle is employed. Preferably a 25 gauge, more preferably a 30 gauge needle is used. In general, approximately 1-2 ml. are required for each cosmetic repair site (e.g. each wrinkle), although repair of large areas may require larger volumes. However, it is generally best to prepare twice this amount since the exact volume to be filled is difficult to calculate. Injection of the cho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com