Method and preparation for reducing irritation and/or inflammatory reaction in human skin
a technology of inflammatory reaction and skin irritation, which is applied in the field of preparation for reducing can solve the problems of considerable discomfort, irritation and/or inflammatory reaction, and complain of skin irritation, and achieve the effect of reducing the occurrence of irritation and/or inflammatory reaction in human skin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
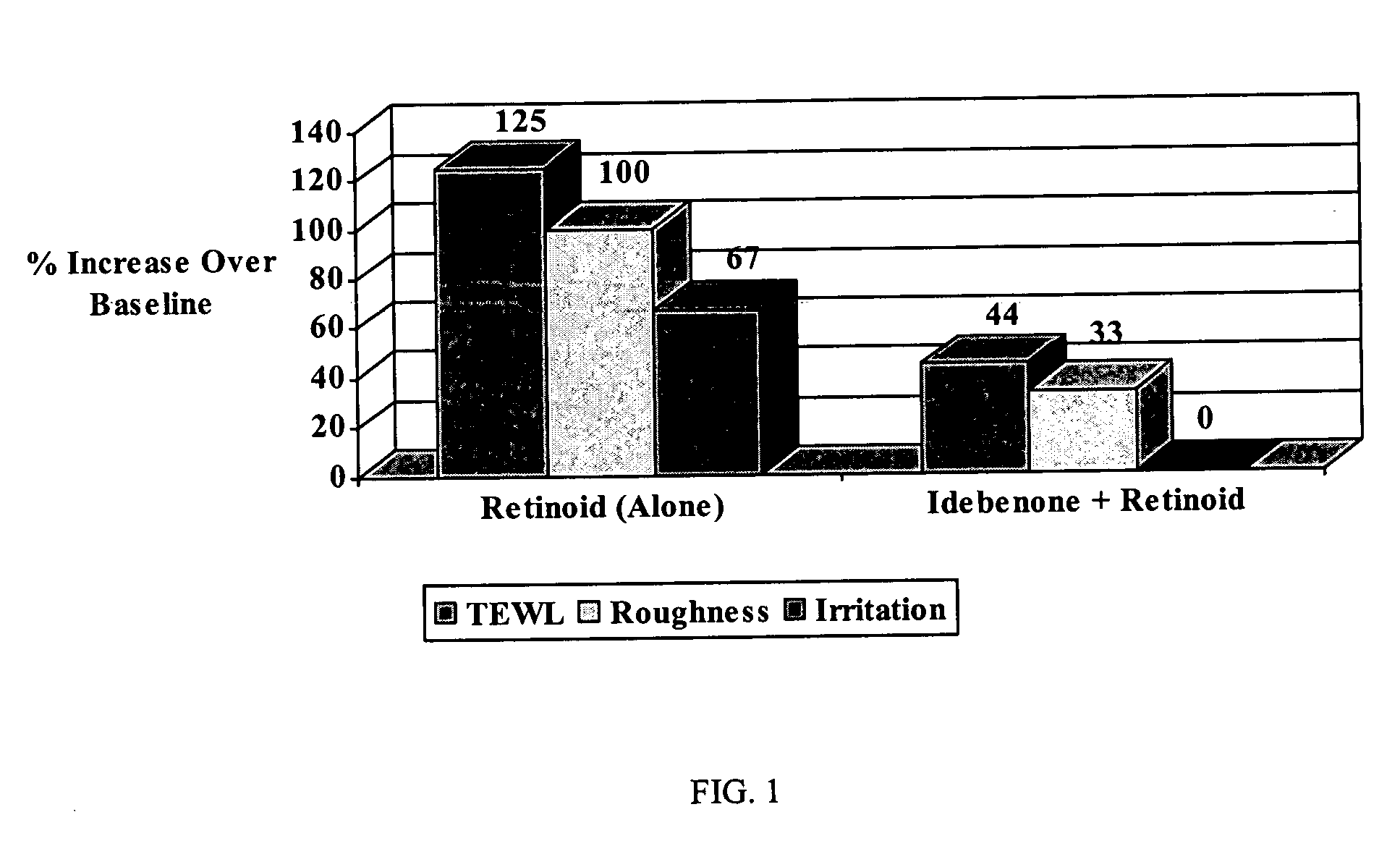
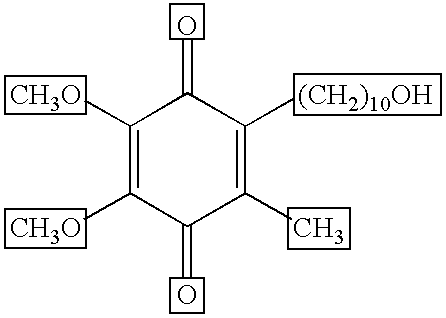
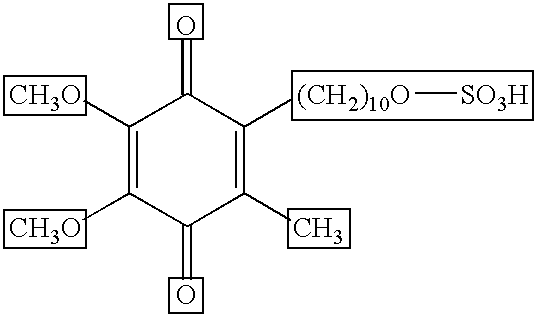
[0067] In a test, idebenone was applied to the facial skin of humans. Two different test sites were setup whereby, one site was applied with idebenone twice a day for 2 weeks followed by a 2 week application period of a prescription retinoid (once a day, as prescribed). The remaining site was applied for 2 weeks just the prescription retinoid (once a day, as prescribed). Each test site was evaluated by a trained clinician for irritation and roughness using a 0 (none) to 4 (severe) scale pre and post retinoid treatment. Additionally, transepidermal water loss measurements (TEWL) were also made at the same time as visual evaluations. At the end of the treatment period, the site treated only with the prescription retinoid exhibited significant increases in TEWL, irritation and roughness compared to a moderate to minimal increase in the same parameters measured when the site was treated with Idebenone for 2 weeks prior to retinoid applications.
[0068]FIG. 1 graphical...
example 2
Methyl Gentisate / Glycolic Acid Irritation
[0070] In another test, idebenone was applied to the backs of humans. Approximately 0.2 ml of a two formulas, one containing Methyl Gentisate and Glycolic Acid and the other the same combination of Methyl Gentisate and Glycolic Acid with 0.5% idebenone. Both were applied to a 2 cm×2 cm square of Webril cotton fabric (affixed to Scanpor semi-occlusive surgical tape) and placed on the back of each subject between the scapulae and waist, adjacent to the spinal mid-line. Patches were removed 24 hours after each application and the test site was allowed to rest for an additional 24 hours prior to repatching. All test sites were graded for erythema and edema prior to reapplication of the next patch. Patch testing was stopped upon the development of moderate irritation at the test sites. The combination of Methyl Gentisate and Glycolic Acid alone produced moderate irritation with edema after 1 patch in 4 out 100 subjects, whereas the combination of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Exposure limit | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com