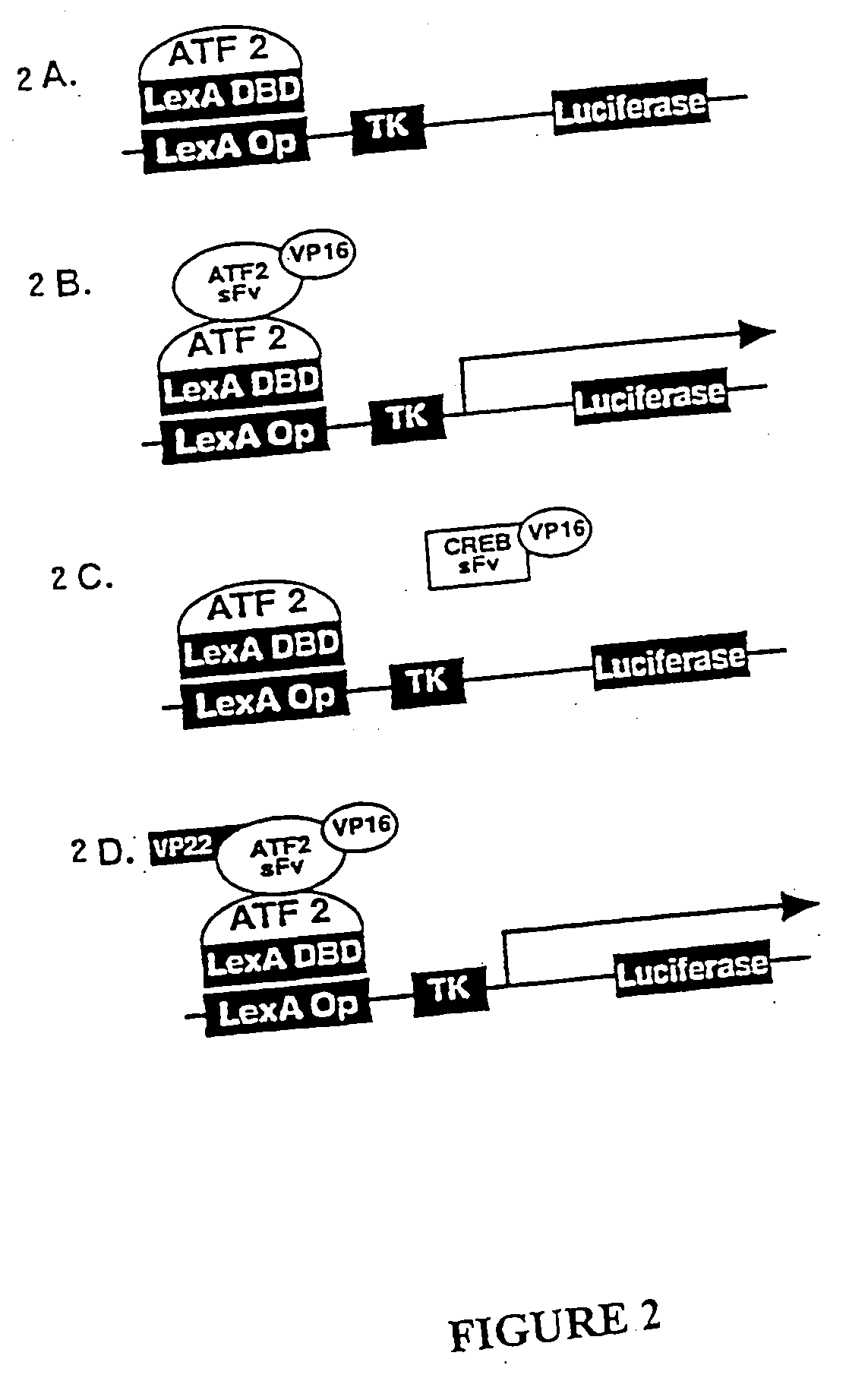
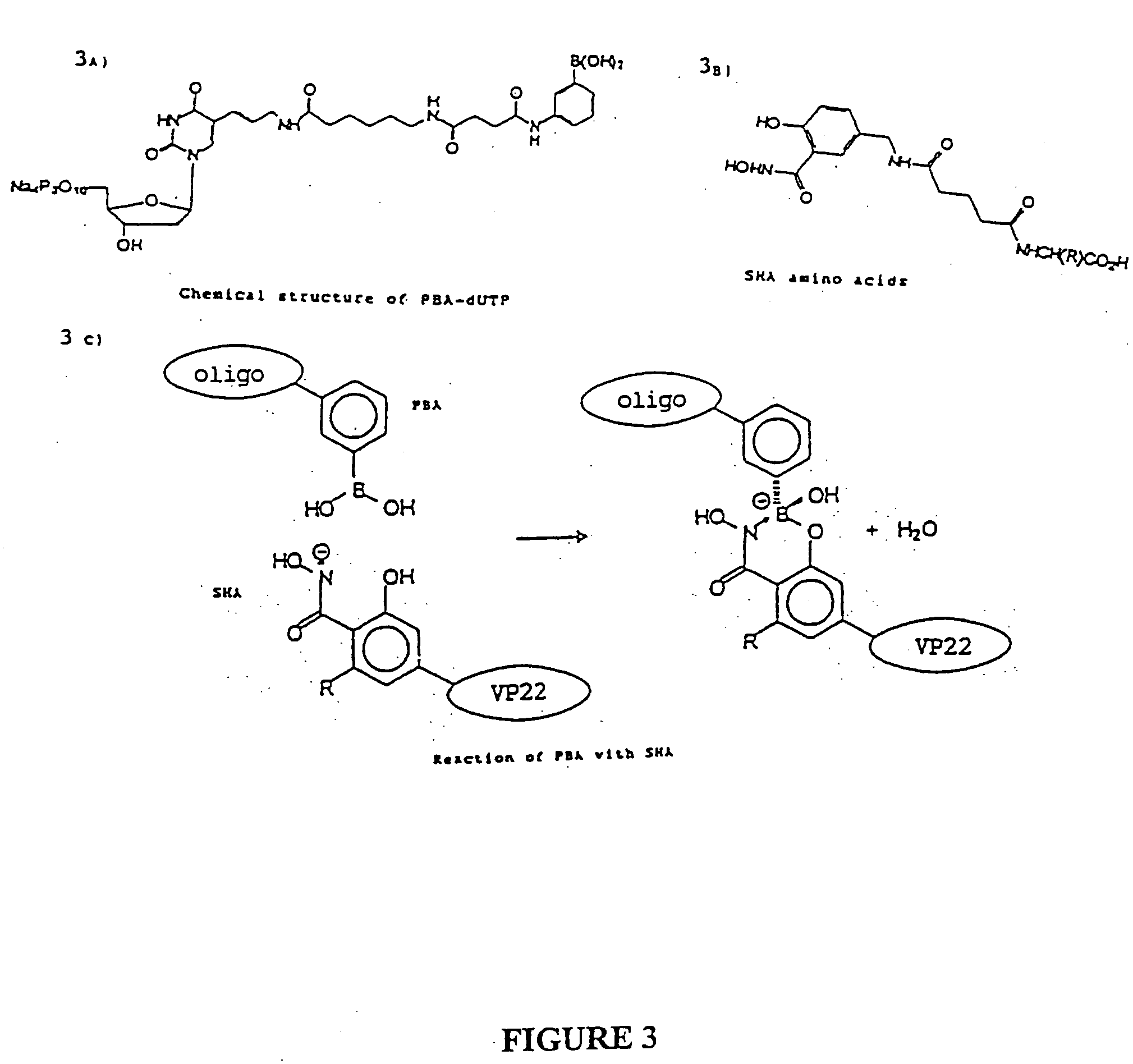
Delivery of functional protein sequences by translocating polypeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Introduction of VP22 Fusion Protein into Cells in Culture by Transfection
[0085] The complete open reading frame (ORF) encoding the VP22 protein was cloned into the eukaryotic expression vector pcDNA3.1 / myc-His (Invitrogen, San Diego, Calif.), to create the vector pVP22 / Myc-His FIG. 5; SEQ ID NO:1), in which the ORF of the fusion partner can be inserted into a multiple cloning site located between the VP22 ORF and sequences encoding the C-terminal Anti-myc epitope and a poly His tag. The anti-myc epitope provides for easy detection of recombinant protein with Anti-myc antibody, and the poly His tag is useful for purification. Alternatively, the vector used was modified by covalent coupling of the Vaccinia Virus Topoisomerase I protein to linearized vector DNA (e.g., pVP22 TOPO® TA Cloning® Kit (Invitrogen)). In this type of vector, the ORF of a gene product of interest (i.e., a “fusion partner”) was cloned as a PCR product into the vector. An example of such a Topoisomerase-adapted...
example 2
Transfection of Cells with a Gene Fusion Followed by Mixing with Untransfected Cells
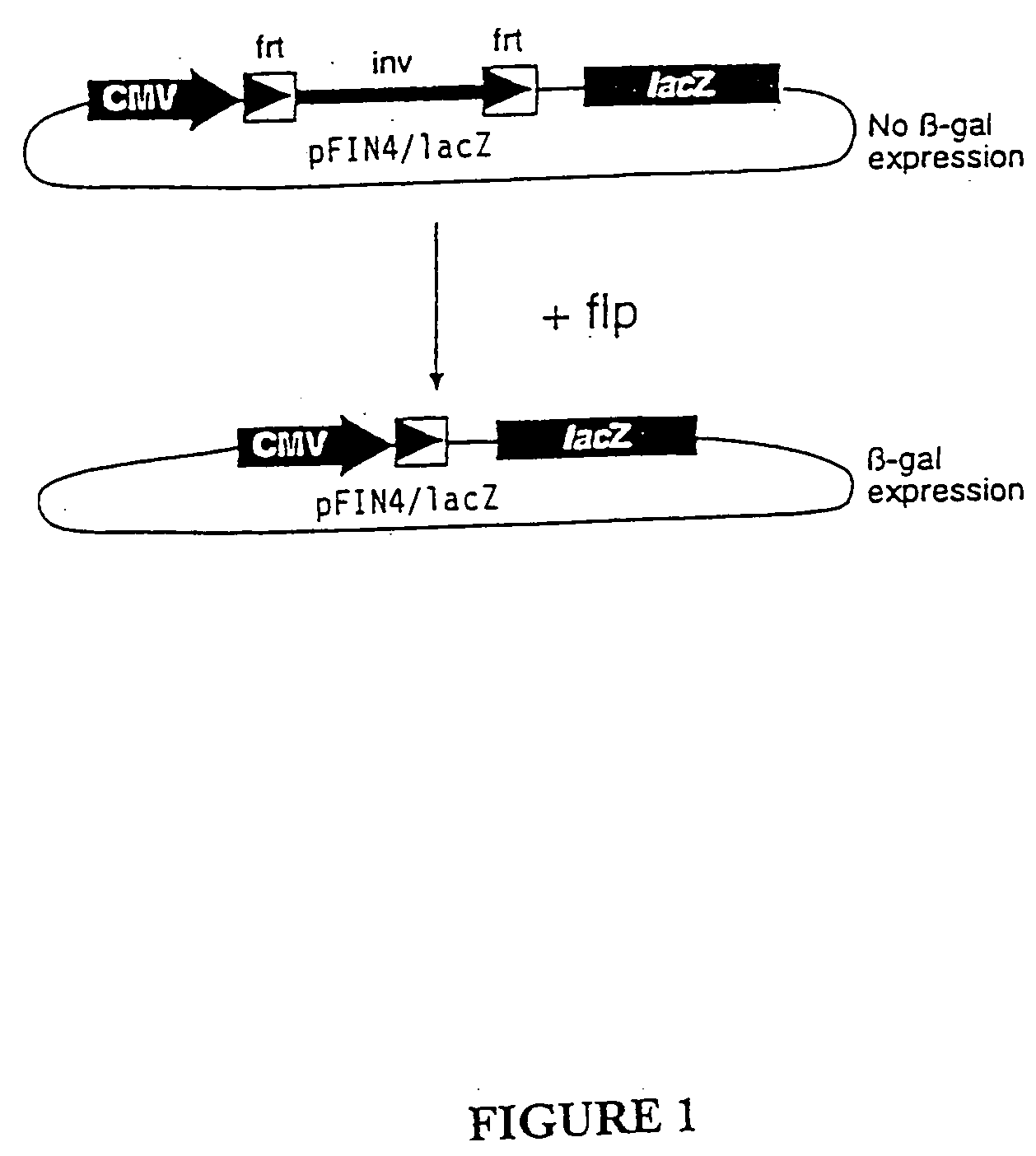
[0089] To demonstrate how VP22 may be used to modulate expression of a functional gene product, a system for delivery of the site specific DNA recombinase Flp, was developed. COS cells expressing a VP22-Flp recombinase fusion protein were prepared as described above and mixed with CHO cells that had been transfected with a reporter plasmid pFIN4 / lacZ (FIG. 1). In the reporter plasmid, a segment of DNA that includes a transcriptional terminator, the Bovine Growth Hormone polyadenylation signal (Goodwin and Rottman, J. Biol. Chem. 267:16330-4, 1992), is flanked by frt sites (recombination sites recognized by the recombinase Flp) to separate the CMV promoter and an otherwise operatively associated reporter gene encoding β-galactosidase as reporter. Cells transfected with pFIN4 / lacZ did not express β-galactosidase due to the presence of the transcriptional terminator placed between the frt sites.
[0090...
example 3
Transfection of Cells with a Gene Fusion Followed by Preparation of a Cell Free Lysate from the Transfected Cells
[0093] In these studies, a cell free lysate was prepared from cells transfected with pVP22 / myc-His as follows: COS cells were grown to 50% confluence in a 100 mm dish (approximately 106 cells). Cells were transfected with 20 μg of pVP22 / myc-His DNA using Pfx-6. Forty hours post-transfection, the cell monolayer was washed twice with PBS and then collected by scraping into 10 ml PBS. Cells were centrifuged at 500 g for 5 min and the PBS was aspirated from the cell pellet, which was then frozen on dry ice. Frozen cell pellets were stored at −80° C. prior to preparation of lysates. The cell pellet was thawed on ice following addition of 0.5 ml ice cold lysis buffer (10 mM HEPES, pH 7.9, 400 mM NaCl, 0.1 mM ethylene diaminetetraacetic acid (EDTA), 0.5 mM dithiothreitol (DTT), 5% glycerol). The lysate was then vortexed briefly and centrifuged at 10000×g for 5 min at 4° C.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
| Stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com