Processes and reagents for oligonucleotide synthesis and purification
a technology of oligonucleotide and reagents, applied in the field of process and reagents for oligonucleotide synthesis and purification, can solve the problems of time-consuming deprotection, purification and analysis procedures, and many research efforts hampered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
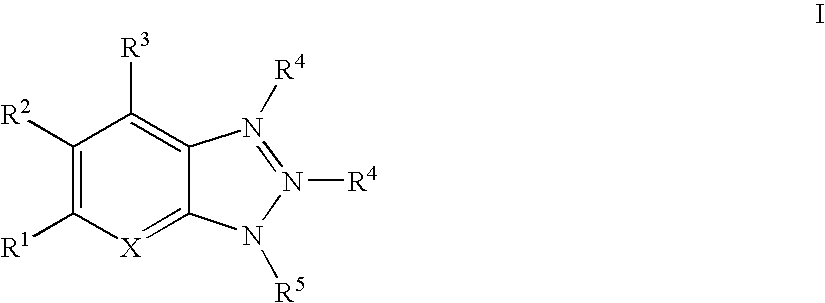
Image
Examples
example 1
Oligonucleotide Synthesis Using Phosphoramidite Activators 35-48 (see FIGS. 1-3)
[0534] In certain instances the strength of the activator is increased by forming the activated salt resulting in decreased coupling time for RNA Synthesis.
[0535] A decamer RNA molecules (49, 5′-CAUCGCTGAdT-3′) was synthesized on a 394 ABI machine (ALN 0208) using the standard 98 step cycle written by the manufacturer with modifications to a few wait steps as described below. The solid support was controlled pore glass (CPG, prepacked, 1 μmole, 500, Proligo Biochemie GmbH) and the monomers were RNA phosphoramidites with fast deprotecting groups obtained from Pierce Nucleic Acid Technologies used at concentrations of 0.15 M in acetonitrile (CH3CN) unless otherwise stated. Specifically the RNA phosphoramidites were 5′-O-Dimethoxytrityl-N6-phenoxyacetyl-2′-O-tbutyldimethylsilyl-adenosine-3′-O-(β-cyanoethyl-N,N′-diisopropyl)phosphoramidite, 5′-O-Dimethoxytrityl-N2-p-isopropylphenoxyacetyl-2′-O-tbutyldimeth...
example 2
Synthesis of compound 1 (R′=H and R″=C(S)OEt or R′,R″=H)
[0538]
[0539] A solution of chlorocarbonyl sulfenyl chloride (8.4 mL, 0.1 mol) in dry ether (50 mL) was added dropwise to a cold solution of thiourea (7.62 g, 0.1 mol) in dry ether (500 mL) and triethylamine (14 mL, 0.1 mol) cooled with ice-bath in 3 h under an argon atmosphere. The reaction mixture was stirred at the same temperature for total of 6 h. The solids were filtered off and the filtration was concentrated into a crude residue which was further crystallized with dichlorometrhane-hexanes to give a pure compound (2.5 g). The mother liquid was then concentrated into a crude residue which was applied to a column of silica gel eluted with dichloromethane-metahnol (40:1) to give a pure compound (180 mg). The total yield is about 30%. 1H-NMR (CDCl3, 400 MHz): δ 10.46 (br, 1H), 4.38 (q, 2H, J=6.8, 14.4 Hz, CH2), 1.39 (t, 3H, J=7.2 Hz, CH3). 3C-NMR (CDCl3, 100 MHz): 181.01, 177.00, 153.75, 64.68, 14.32.
example 3
Phosphorothioation of Di- and POLY-Oligothymidine Using Sulfur Transfer Reagent 1 (R′=H and R″=C(S)OEt or R′,R″=H):
[0540] Dinucleotide 2 and hexamer 3 were synthesized on a 394 ABI machine using the standard 93 step cycle written by the manufacturer with modifications to a few wait steps as described below. Activator used was 5-(ethylthio)-1H-tetrazole (0.25 M), and for PS-oxidation, 0.05 M 1 in anhydrous acetonitrile was used. The sulfurization time was about 4 min. After completion of the synthesis, 2 and 3 were deprotected from support by aqueous ammonia treatment at 55° C. for 1 h. After HPLC purification, the compound were analysed by LC-MS.
[0541] The results of phosphorothioation of oligothymdine using 1 as the sulfur-transfer agent are shown below.
Sequence,MassMassCompoundall P═SCalc.Found25′ TT 3′562.46562.2235′ TTTTTT 3′1843.521842.05
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Equivalent mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com