Bioprosthetic heart valves
a heart valve and bioprosthetic technology, applied in the field of bioprosthetic heart valves, can solve the problems of significant tissue calcification, high cost, and high cost, and achieve the effects of improving overall human health care, and reducing the risk of heart valve failur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effects of Hyperlipidemia on Heart Valve Tissue
[0065] Rabbits were used since they have a propensity to develop endothelial dysfunction when subjected to a cholesterol diet. The effects of feeding New Zealand White Rabbits a diet supplemented with 1% cholesterol (wt / wt; Purina Mills, Woodmont, Ind.) were studied. Briefly, the rabbits were fed cholesterol for ten weeks, while control rabbits received a normal diet. After ten weeks, the rabbits were anesthetized using intramuscular ketamine (68 mg / kg), xylazine (9 mg / kg), and aceprmazine (2.3 mg / kg). Heparin (100 U / kg) was administered intracardially, and the rabbits were killed four to five minutes later with an overdose of a pentobarbital sodium solution. The thorax was opened widely, and the heart removed for histologic, RT-PCR, and immunostaining analysis of the aortic heart valve. After removal, the aortic heart valves were either frozen in liquid nitrogen, or fixed in 10% formalin and embedded in parafilm. The valvular appearan...
example 2
Effects of HMG CoA Reductase Inhibitors and Oxidative Stress on Heart Valve Tissue In Vitro
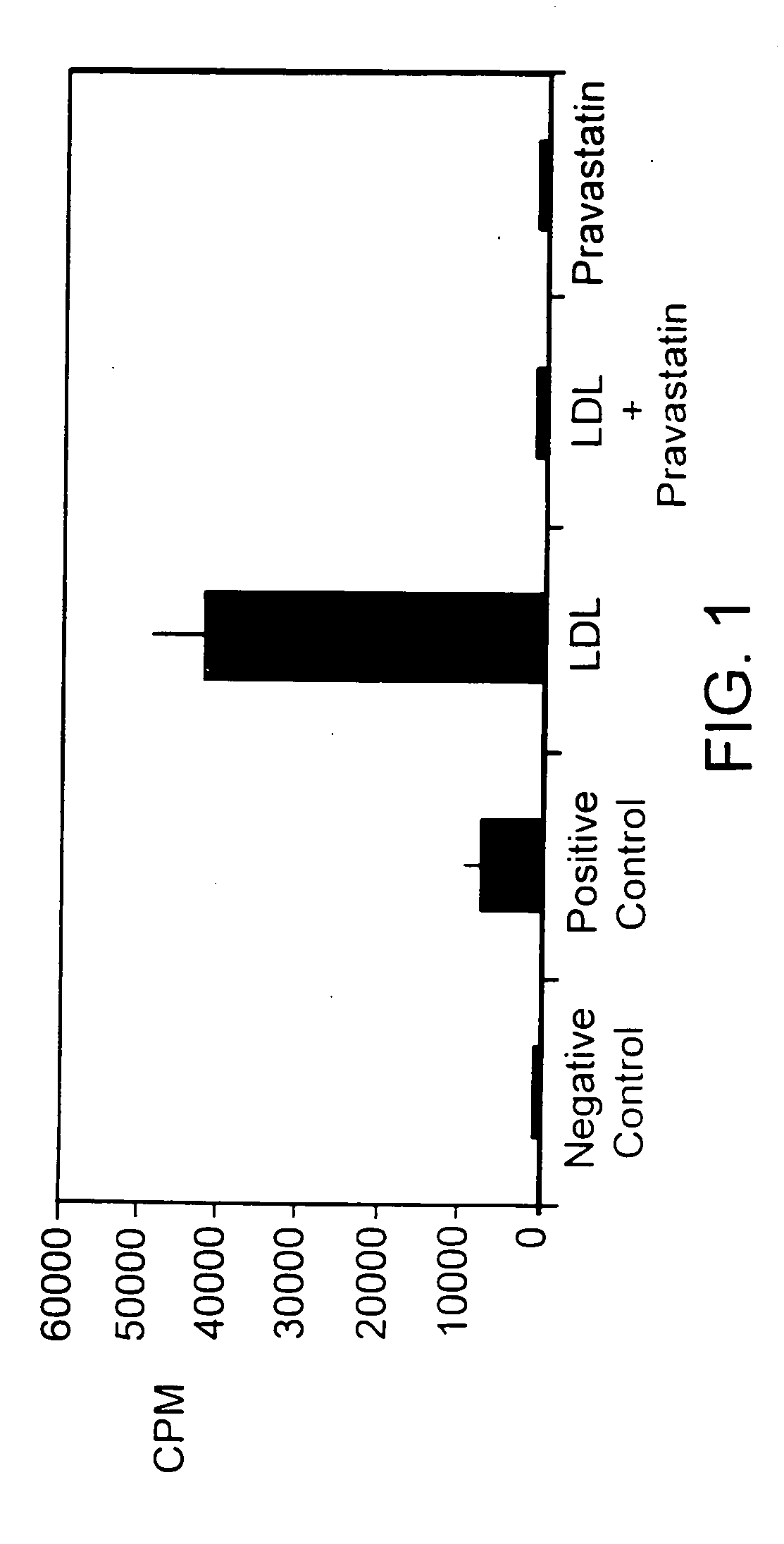
[0071] Valvular endothelial and subendothelial tissues were obtained from mature pigs and used to create primary explant cultures. Briefly, the cells were isolated from the cardiac aortic valves by collagenase digestion, and cultured in medium 199 with 10% (v / v) heat-inactivated fetal bovine serum at 37° C. in a humidified atmosphere of 5% CO2 in air. For each experiment, the cells were used between the 3rd and 7th passage. Cells were grown to about 80 percent confluence in 24-well plates. Once at about 80 percent confluence, the cells were incubated in serum-free medium for 24 hours to arrest growth. After the 24 hour incubation, LDL (10 μg / mL) alone, pravastatin (100 nM) alone, or LDL (10 μg / mL) plus pravastatin (100 nM) was added to the wells, and the cells incubated for 18 hours. Controls were cells treated with either serum-free media or media containing 10% fetal bovine serum. Pravastat...
example 3
Adenoviral Vectors
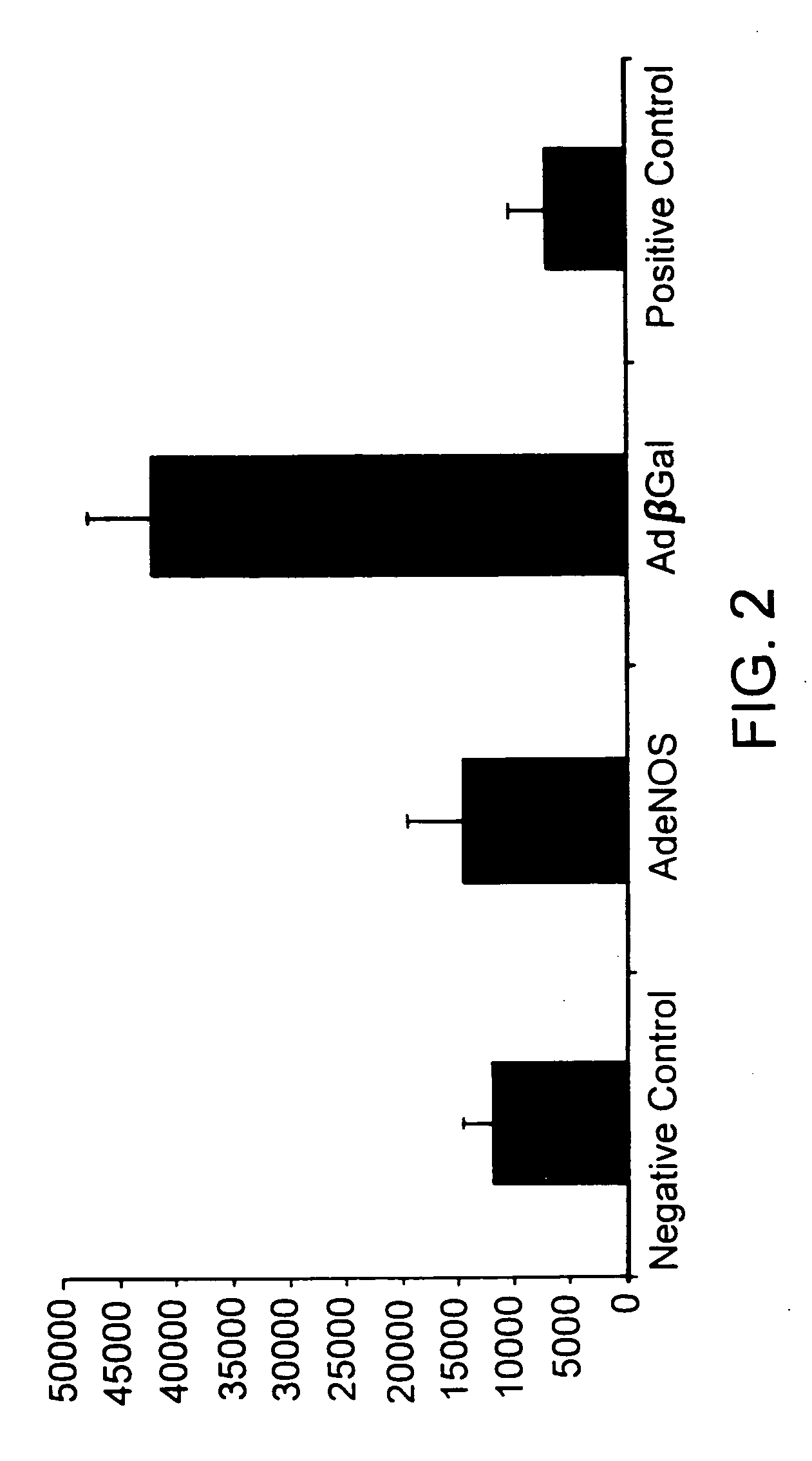
[0077] Adenoviral vectors containing a cDNA encoding either β-galactosidase or eNOS were constructed using methods similar to those described by Tsutsui et al. (Arterio. Thromb. Vascul. Biol. 18:1231-1241 (1998). Briefly, each vector was produced by cloning the cDNA of interest into a plasmid containing a subsegment of the adenoviral genome. Each resulting construct then was cotransfected into human E1a-293 cells along with adenoviral DNA containing an E1a deletion. Adenoviruses containing a E1a deletion are replication defective, however, viral replication can occur within E1a-293 cells since these cells provide E1a products in trans. Through homologous recombination, recombinant adenoviral vectors containing the cDNA of interest were produced within the 293 cells. The recombinant adenoviral vectors were isolated and propagated. Once collected, each adenoviral vector stock was stored at −70° C. in 0.01M Tris, 0.01M MgCl, and 10% glycerol. The recombinant adenovir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com