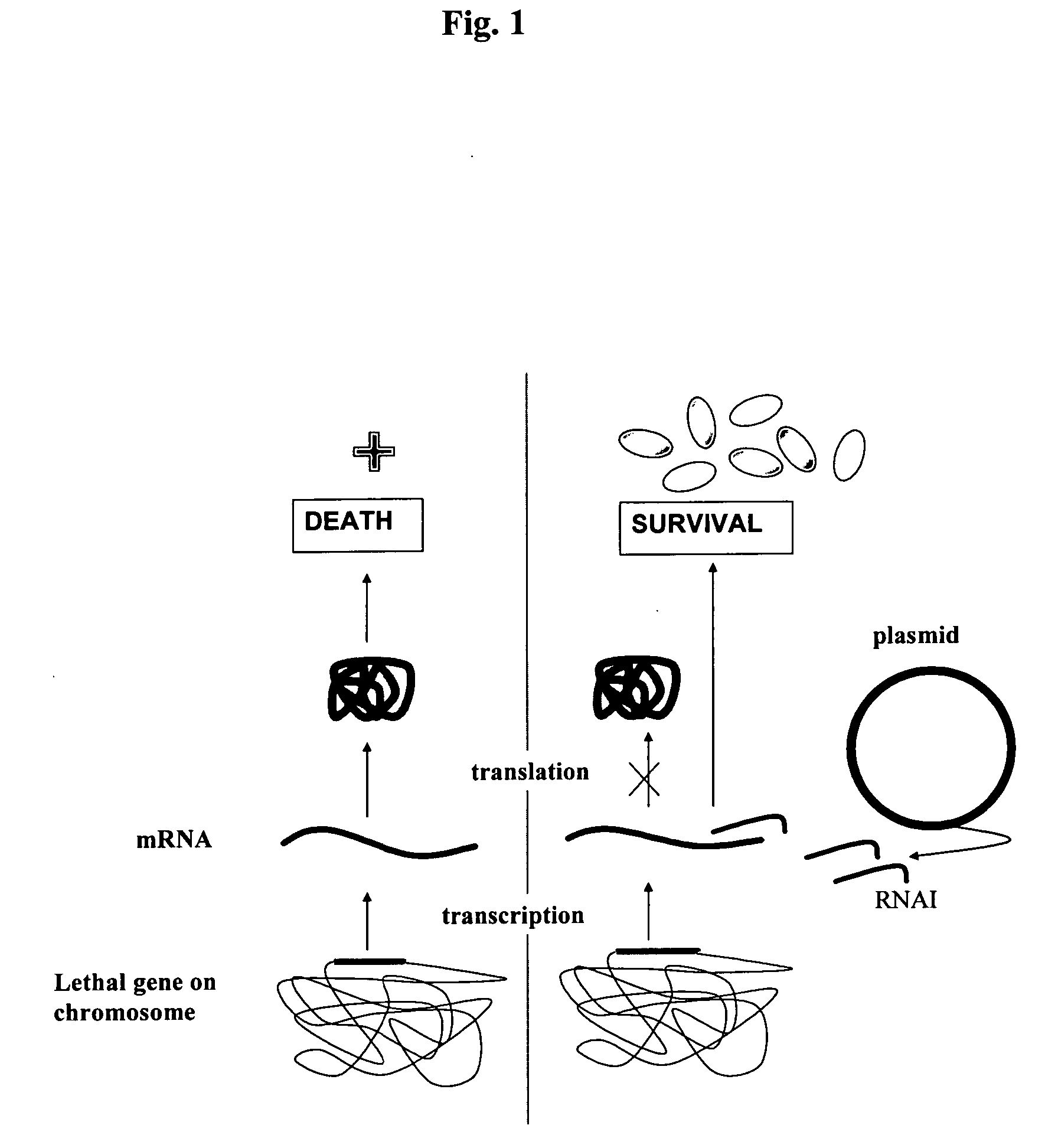
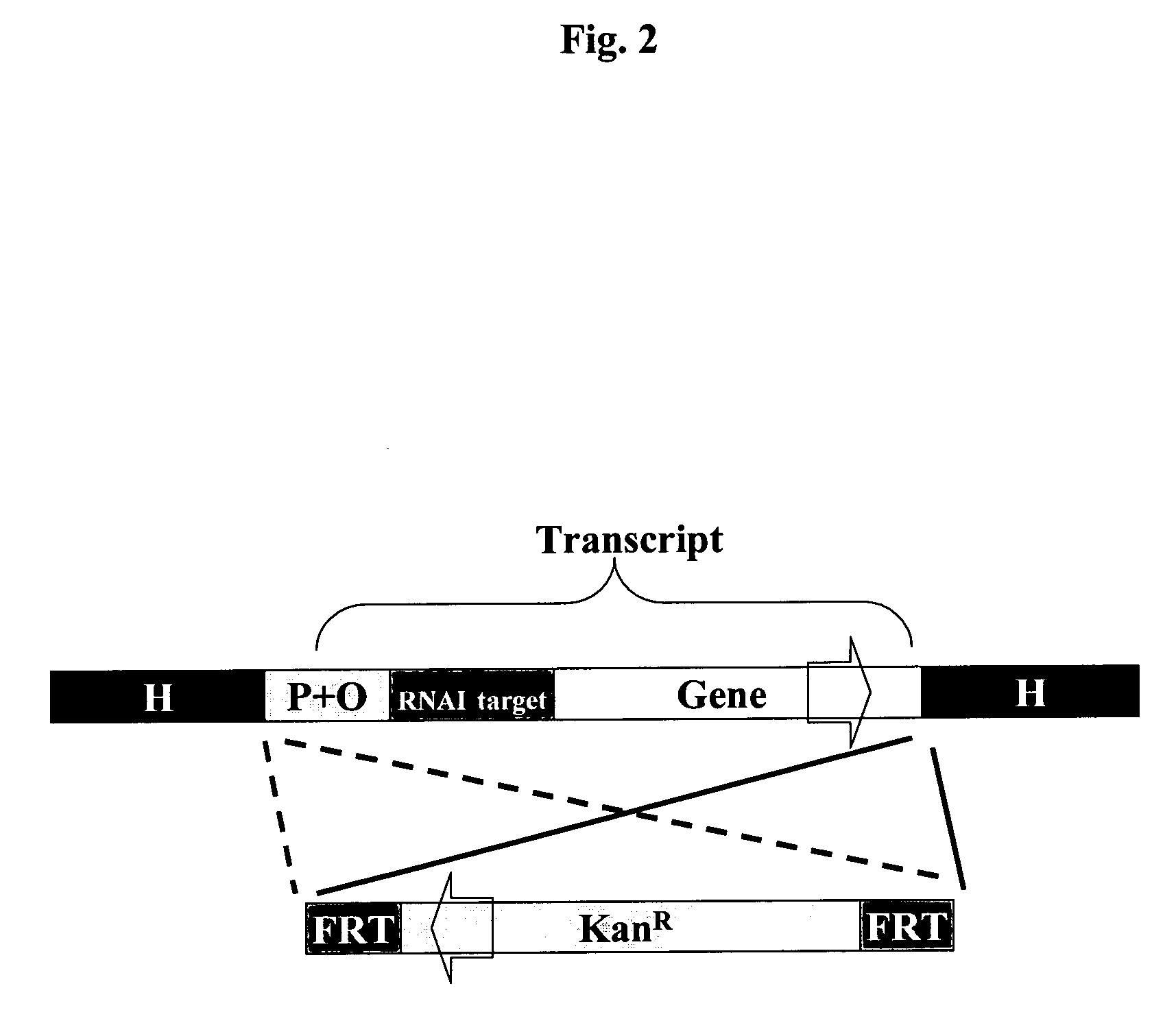
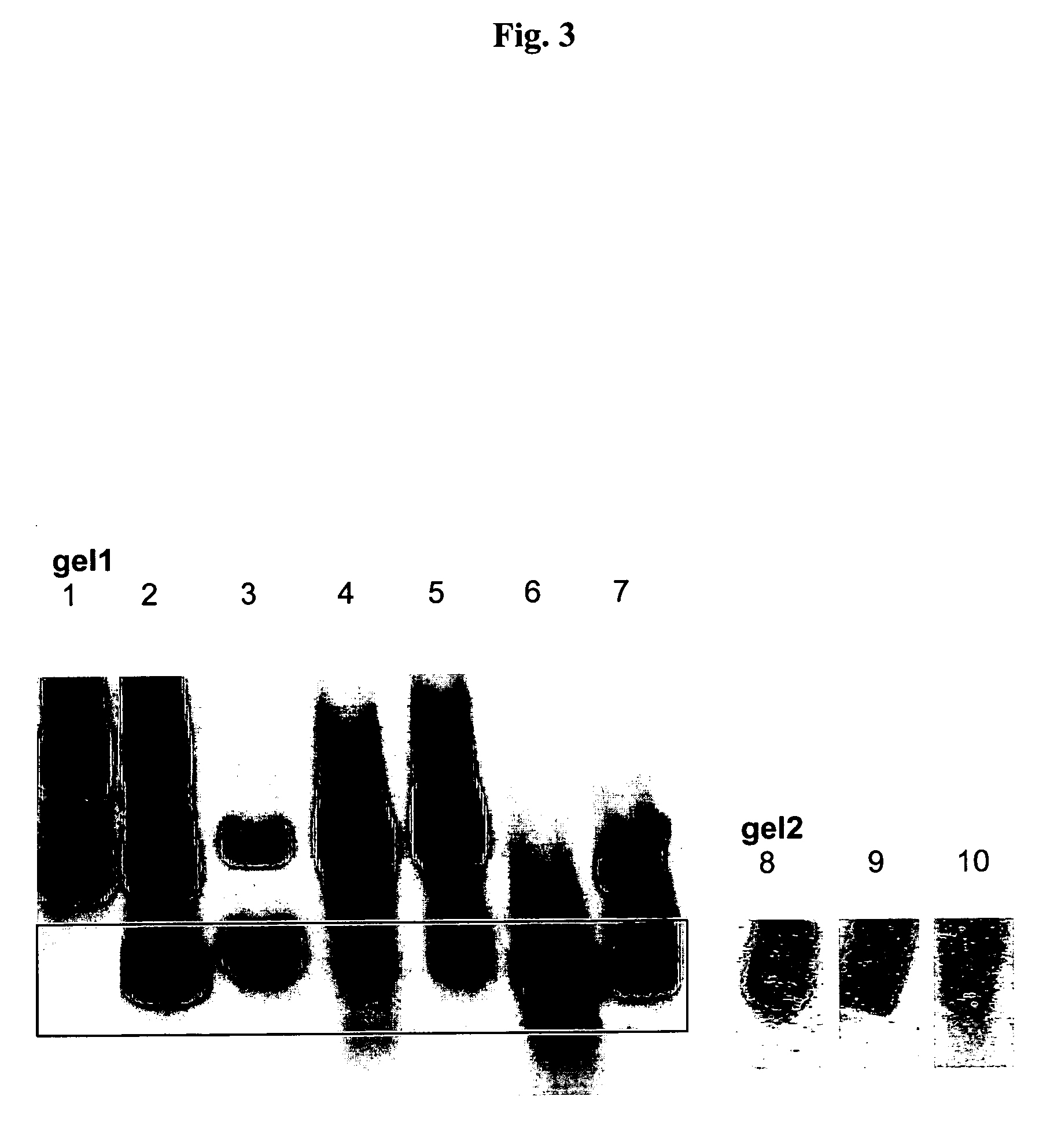Host-vector system for antibiotic-free CoIE1 plasmid propagation
a coie1 plasmid and host technology, applied in the field of plasmid dna as gene transfer vehicle, can solve the problems of inconvenient use of antibiotics, additional genes on the plasmid, and potential contamination of the final product with residual antibiotics, and achieve the effect of inhibiting transcription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3
[0158] Expression / Suppression of Marker Gene During Fermentation
[0159] The E. coli strains IS11 and IS5 are analyzed during a fed-batch fermentation process, with and without the presence of plasmid pBR322. Table 4 summarizes the experimental set-up of four fed-batch fermentations. Each strain is grown either in the presence or absence of pBR322.
TABLE 4Fed batch fermentationsExperimentHost strainpBR322AS1IS 11−AS2IS 11+AS3IS 5−AS4IS 5+
[0160] All four cultivations show very similar trends for online signals such as CO2, O2, base consumption or capacity and the course of total BDM varies also in a very small range of less than ±10% from the calculated mean as shown in FIGS. 7a and 7b. FIG. 7a shows bacterial dry mass (BDM) and GFP expression of IS II with or without maintenance of pBR322. While the total BDM is identical for both fermentations, the GFP concentration is drastically decreased when pBR322 is present (50%). The curve progression of GFP measurements strongly indicates i...
example 4
[0161] Use of a Repressor for Regulating the Expression of an Essential Gene
a) Generation of Constructs for Essential Genes
[0162] The first essential gene to be tested is map (Li et al., 2004), the gene for the methionine aminopeptidase, which is located at min 4 of the E. coli chromosome, 357 base pairs from the rpsB-tsf operon and 201 bp from the T44-RNA gene. The two genes are transcribed divergently and promoters do not overlap. This is an essential point, because the promoter of the essential gene is to be removed entirely and replaced by an inducible promoter that is specific for a chosen repressor. Chang et al, 1989 described a conditionally lethal mutant strain which has the map gene controlled by the lac promoter. By the map cassette, a 67 bp chromosomal section is replaced containing the map promoters (Chang et al, 1989). To circumvent possible transcripts from the genome, two strong transcriptional terminators T1 and T2 from the rrnB operon (Brosius et al, 1981) are ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Toxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com