Carbon monoxide dependent guanylyl cyclase modifiers and methods of use
a guanylyl cyclase and carbon monoxide technology, applied in the direction of biocide, immunological disorders, drug compositions, etc., can solve the problems of inability to effectively compensate for loss, reduced acetylcholine binding sites, etc., to achieve the effect of increasing acetylcholine binding sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
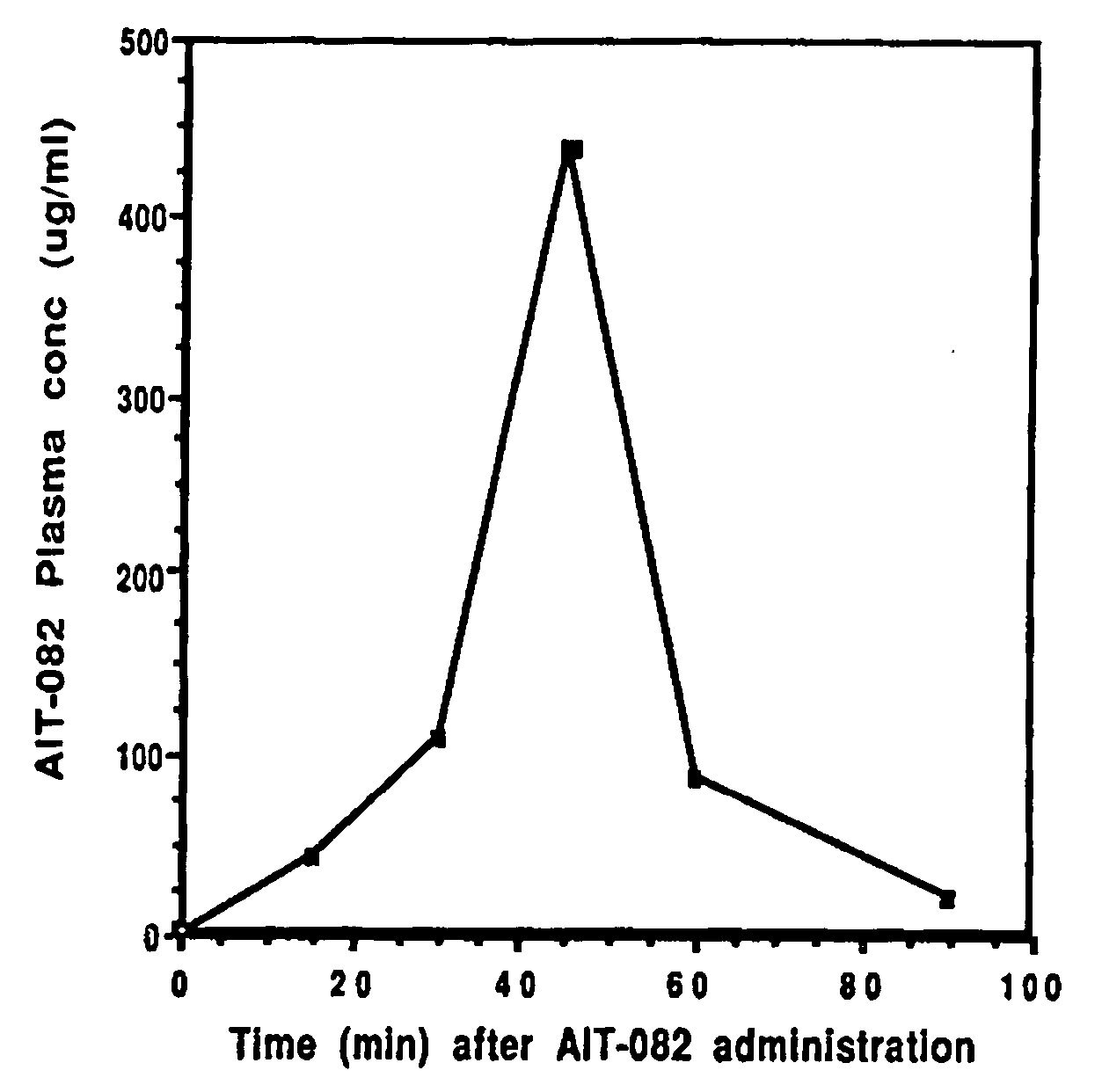
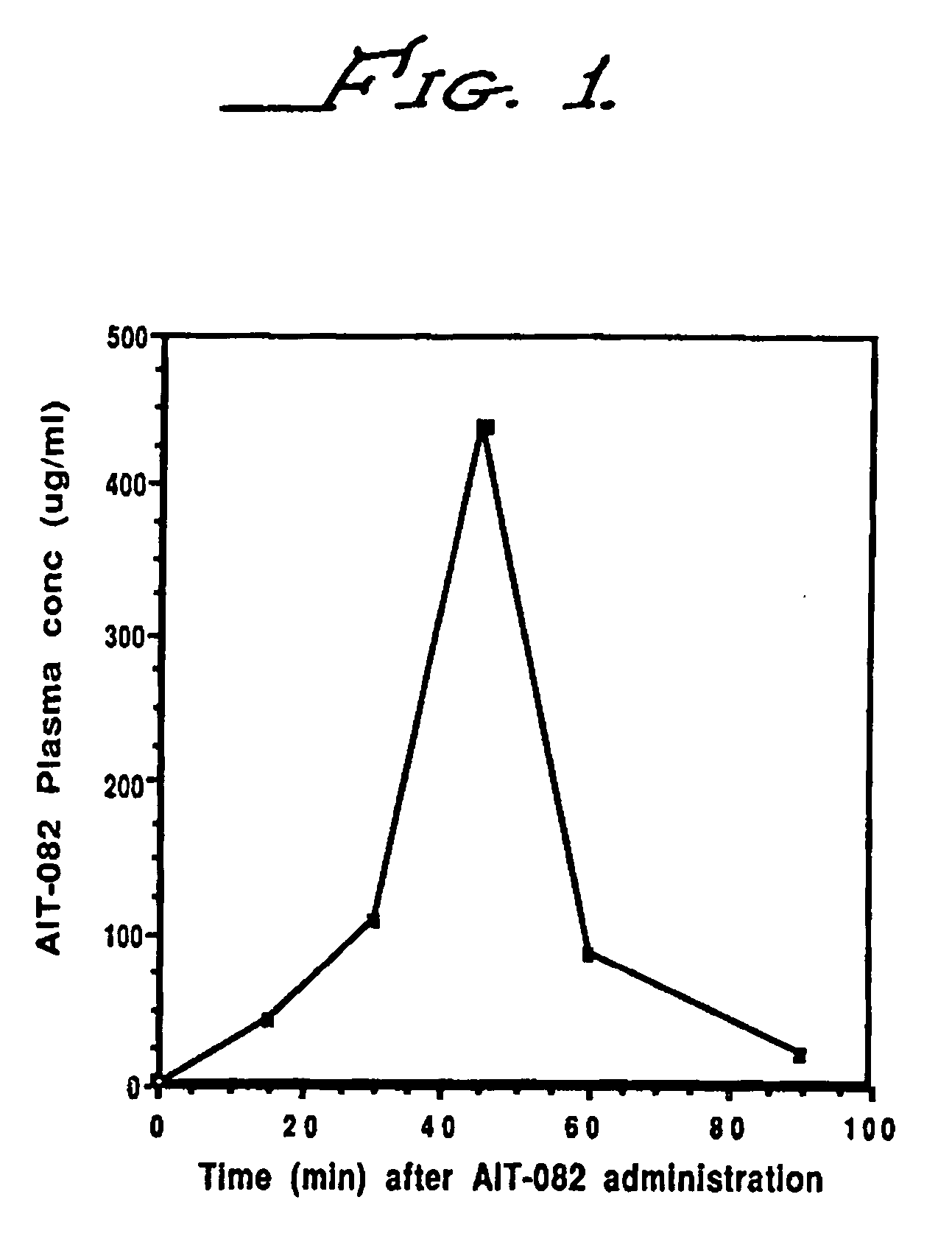
Plasma Levels of AIT-082 in Mice
[0069] Adult C57BL / 6 mice were administered 30 mg / kg of AIT-082 in saline i.p. The animals were sacrificed by decapitation at 30, 45, 60 and 90 minutes after administration of AIT-082. Blood was collected in heparinized tubes, mixed and centrifuged at 2000 rpm for 15 minutes. The plasma supernatant was removed and stored at −70° C. until analysis. A high pressure liquid chromatography system was developed for the analytical measurement of AIT-082 in plasma and brain tissue. The assay developed was selective for AIT-082 in the presence of a number of closely related purine molecules. The sensitivity of the method was 0.1 microgram of AIT-082 per ml of plasma and 0.1 microgram of AIT-082 per milligram of brain tissue (wet weight).
[0070] The results of these determinations are shown in Table A and graphically represented in FIG. 1 where plasma levels of AIT-082 are provided at 30, 45 and 60 minutes after administration of 30 mg / kg i.p. to C57BL / 6 mice....
example 2
AIT-082 Crosses the Blood Brain Barrier
[0071] Brain tissue was analyzed from two animals receiving 30 mg / kg i.p. of AIT-082 and sacrificed 30 minutes after drug administration. The brains were rapidly removed and chilled on ice. Brain tissue was dissected into cortex and remainder of the brain. Brain tissue (approx. 250-300 mg wet weight) was homogenized with 5.0 ml of saline using a Brinkman Polytron tissue grinder and stored at −70° C. until analysis. Brain homogenates were deproteinized by ultrafiltration through Gelman Acrodisc filters; first through a 1.2 micron filter and then through a 0.2 micron filter. A 30 μl sample was injected into the HPLC for analysis as above. A standard curve was prepared by the addition of known quantities of AIT-082 to brain homogenates from untreated animals. Analysis of the brain tissue indicated that AIT-082 was detected in both the cortex sample and the remaining brain samples from both animals. The results are shown directly below in Table B....
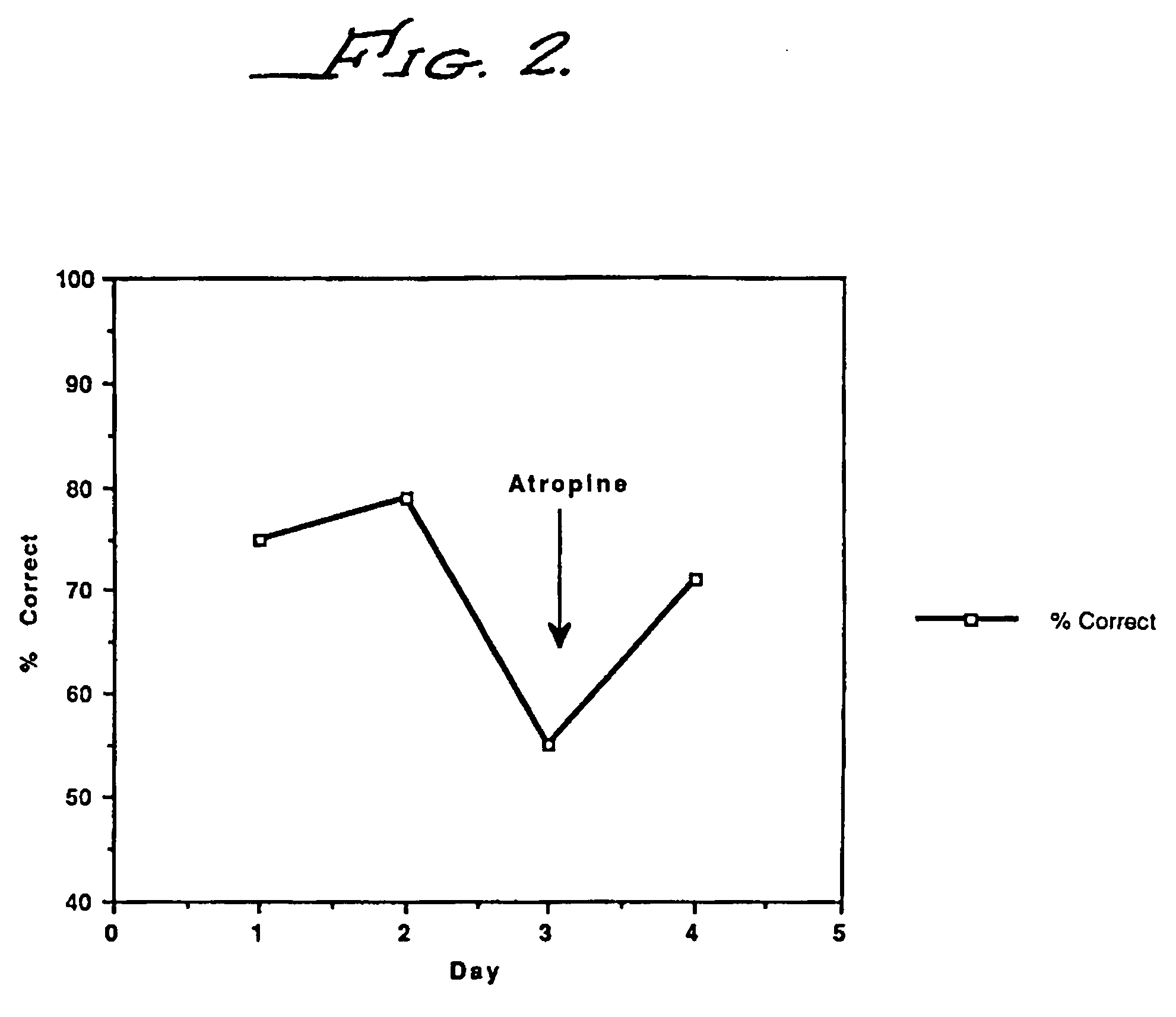
example 3
AIT-082 Interacts with the Cholinergic System
[0073] Because of the finding that there is a severe loss of cholinergic neurons in the hippocampus in Alzheimer's disease patients, there has been considerable interest in the effect on memory of compounds which alter the activity of this system. Support for the cholinergic hypothesis of memory comes from studies using lesions or a stroke model. Lesions of the CA1 region of the hippocampus appear to specifically disrupt working memory. In the stroke model, occlusion of the vertebral and carotid arteries (30 minutes) produces specific cell loss in the CA1 region of the hippocampus and a loss of working memory. In these models in aged rats, physostigmine, a cholinesterase inhibitor, has been shown to improve memory. THA, another drug which increases cholinergic function, was shown to improve memory in aged monkeys. The observation that AIT-082 improves memory in the same general manner as physostigmine and THA raises the question of wheth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com