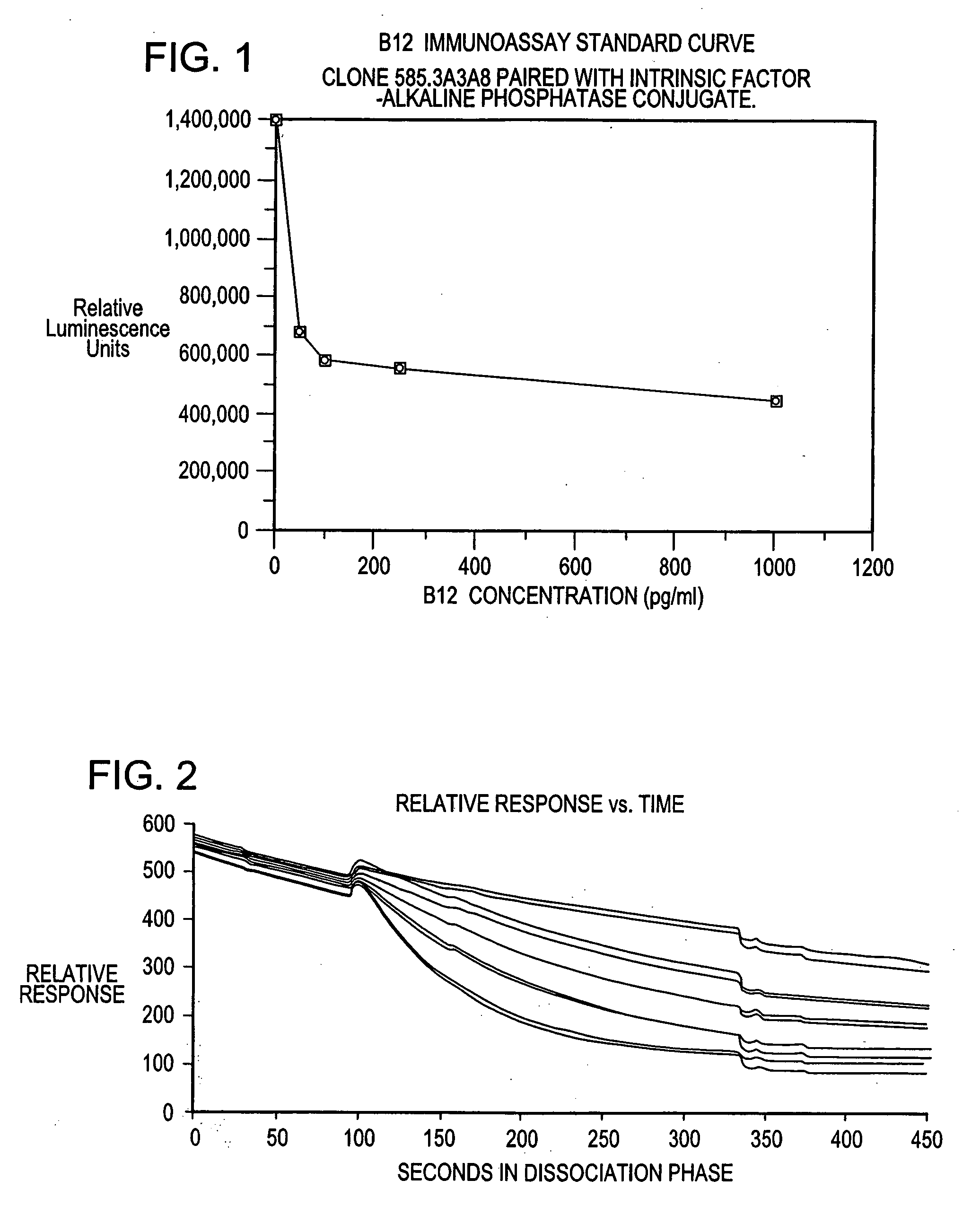
Immunoassays for determining vitamin B12, and reagents and kits therefor
a technology of immunoassays and vitamin b12, which is applied in the field of immunoassays for determining vitamin b12 levels in samples, can solve the problems of affecting the apparent specificity of said preparations, unable to detect the presence of a single molecule, and unable to detect a single molecule, etc., to achieve the effect of flexible and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Antibodies
[0042] Eight week old female BALB / c mice were immunized on a biweekly basis with 10 ug / dose of porcine intrinsic factor in Incomplete Freund's adjuvant by the intraperitoneal route. The mice were sacrificed by cervical dislocation, and the spleens surgically removed. The spleens were transferred to a petri dish containing 5 ml of Dulbecco's modified medium (DME) containing 100 ug / ml gentamycin. The splenocytes were freed by teasing the tissue apart. The splenoctyes were washed once with DME, and combined in a 5:1 ratio with washed SP2 / O cells, from a plasmocytoma cell line that can be obtained commercially.
[0043] The cells were pelleted, excess supernatant removed, and the cells resuspended in 1 ml of buffered 50% polyethylene glycol solution (Boehringer Mannheim). The cells were incubated at room temperature for 120 seconds prior to centrifugation at approximately 400×g for 6 minutes. Fused cells were then centrifuged, gently rinsed with DME, resuspended ...
example 2
Preparation of Enzyme Labelled Antibodies
[0048] Maleimide groups were introduced onto alkaline phosphatase (ALP) by reaction with a 30-fold molar excess of sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexanel-carboxylate in 0.2 M imidazole, pH 9.0; excess reagent was removed by gel filtration on a 1×28 cm column of Sephadex G-50 in PBS. Thiols were introduced onto the antibody (obtained through the procedure described in Example 1) with 40 mM N-acetyl homocysteine thiolactone in 0.1M sodium bicarbonate, 0.15 M NaCl, 1 mM EDTA, pH 8.0; excess reagent was removed on a second gel filtration column in the PBS+1 mM EDTA. Conjugation was achieved by incubating modified enzyme with modified antibody at a 2:1 molar ratio of ALP to antibody for two hours at room temperature. The conjugate was purified by size exclusion chromatography on Sephacryl S-300 (1.5×120 cm) in Tris-buffered saline, pH 8.0.
example 3
Preparation of Enzyme Labelled Intrinsic Factor
[0049] Maleimide groups were introduced onto alkaline phosphatase as above. Purified intrinsic factor (obtained from Scripps Laboratories, Inc.) was reacted with 40 mM N-acetyl homocysteine thiolactone in 0.08 M sodium bicarbonate, 0.12 M NaCl, 8 mM EDTA, pH 8.0; excess reagent was removed on a second gel filtration column in the PBS+1 mM EDTA, as above. Conjugation was achieved by incubating modified intrinsic factor with a two-to-three-fold molar excess of modified enzyme for two hours at room temperature. Under these conditions thiols, such as were introduced into intrinsic factor, are known to react stoichiometrically with maleimides, as on the modified enzyme, to form chemically stable linkages. The conjugate was purified by size exclusion chromatography on Sephacryl S-300 (1.5×120 cm) in Tris-buffered saline, pH 8.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com