5-hydroxytryptophol (5-HTOL) derivatives, antibodies, immunoassays and detection of recent alcohol consumption
a technology of 5hydroxytryptophol and derivatives, applied in the field of antibodies, can solve the problems of expensive instruments and drawbacks of methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
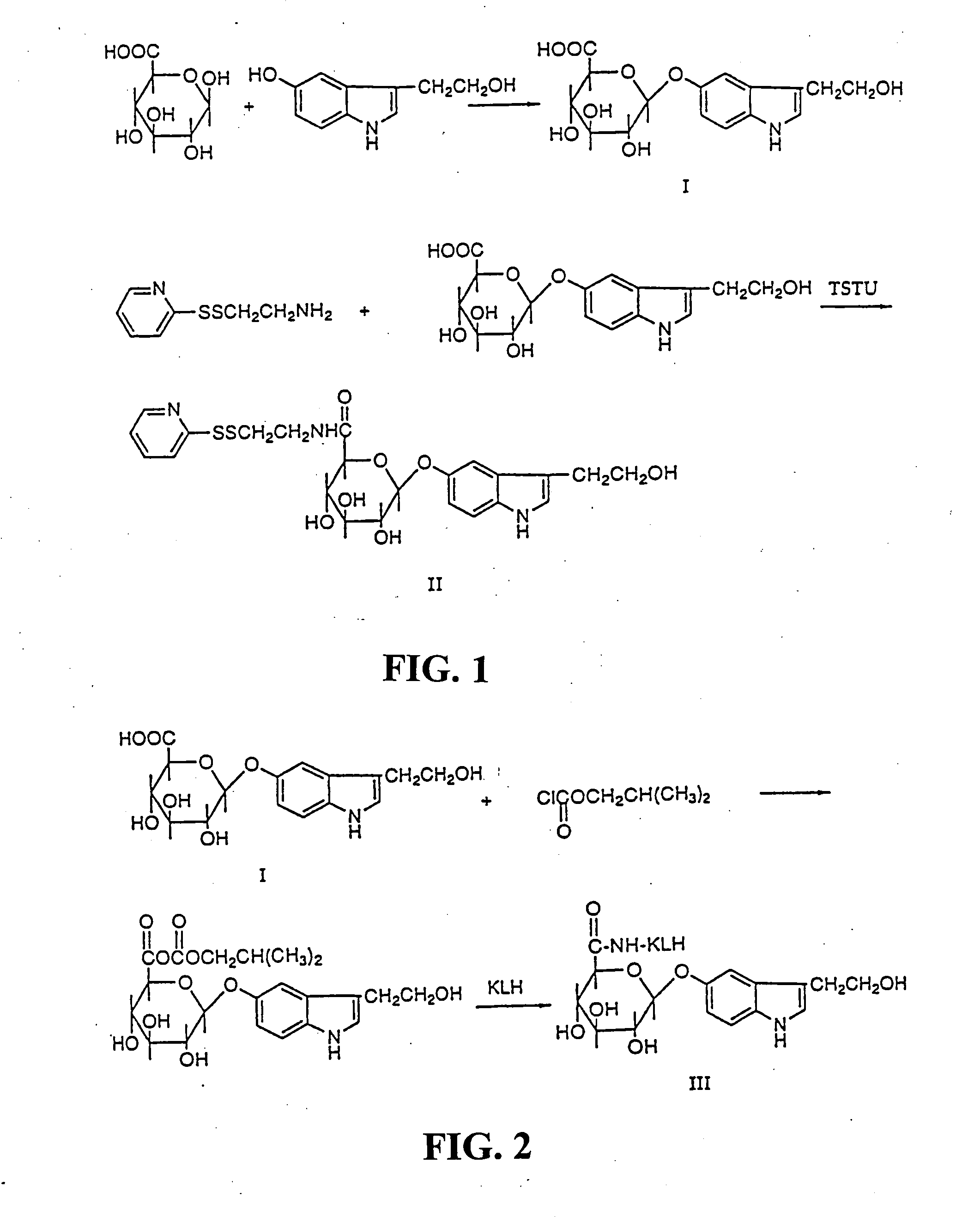
[0032] Synthesis and Purification of 3-(2-hydroxyethyl)indole-5-yl-O-β-D-glucopyranosiduronic acid (I) (Scheme 1): 2-(5-Hydroxy-3-indolyl)ethyl alcohol (151 mg), MgCl2 (50 mg), uridine 5′-diphosphoglucuronic acid triammonium salt (490 mg), uridine 5′-diphosphoglucuronyl transferase (182 mg) (Type III from bovine liver, Sigma) and 40 ml of 0.1 M pH 7.4 Tris-buffer were added to a 200 ml E-flask. The flask was sealed with parafilm and incubated in a shaker-incubator at 37° C. and 150 rpm. Small samples were extracted from the incubation at different timepoints, and analysed after cooling, centrifugation, filtration and acidification by analytical PepRPC on a FPLC system, in order to follow the reaction. The concentration of 3-(2-hydroxyethyl)indole-5-yl-O-β-D-glucopyranosiduronic acid (I) reached plateau-levels after 20 h and remained at that level for more than 7 h.
[0033] The incubation was stopped on ice for 15 min followed by centrifugation at 10000 rpm at 5° C. for 30 min using a...
example 2
[0034] Synthesis of N-(2-pyridyldithioethyl) -(2-hydroxyethyl)indole-5-yl-O-β-D-glucopyranosiduronic acid (II). Scheme 2. 3-(2-Hydroxyethyl)indole-5-yl-O-β-D-glucopyranosiduronic acid (I) (52 mg, 0.153 mmole) was dissolved in 1 ml DMF (dried with molecular sieves) in 5 ml reacti vial. Thereafter O-(N-succinimidyl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TSTU)(73 mg, 0.243 mmole) and diisopropylethylamine (84 μl, 0.456 mmole) were added The reaction was run for 35 min in room temperature.
[0035] Cysteamine-2-pyridyldisulfide hydrochloride (72 mg, 0.324 mmole) was added to another reacti vial followed by DMF (0.5 ml) and diisopropylethylamine (56 μl, 0.324 mmole). To this solution the hydroxysuccinimidyl activated acid (I) was added. The reaction was run for 1 h and then evaporated in vacuum at low pressure giving an oil. TLC (n-butanol:ethyl acetate:acetic acid:water=1:1:1:1) showed that almost all (I) had reacted. The oil was dissolved in 3 ml of 27% methanol, divided in thr...
example 3
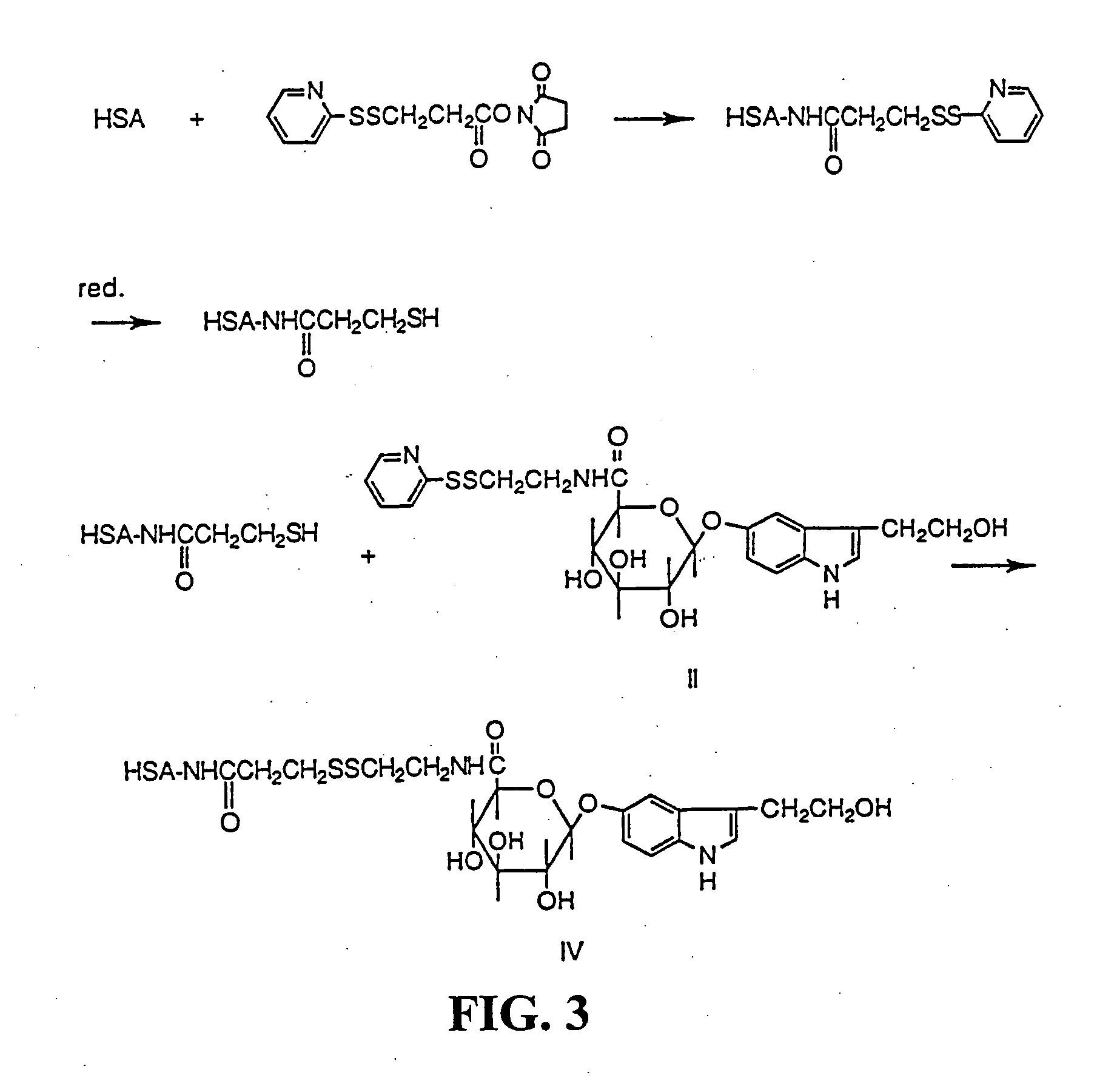
[0036] Synthesis of the KLH conjugate (III) (Scheme 2). Keyhole limpet hemocyanine (KLH) (73.5 mg) was dissolved in 6.5 ml of 0.1 M borate buffer keeping pH 9.5. Undissolved substance was removed by filtration and the protein content of the solution was analysed by amino acid analysis to be 37 mg / ml protein. To this ice-cold solution of KLH 0.8 ml of a solution of a mixed anhydride of 3-(2-hydroxyethyl)indole-5-yl-O-β-D-glucopyranosiduronic acid (0.025 mmole) was added. The latter compound was prepared by dissolving 3-(2-hydroxyethyl)indole-5-yl-O-β-D-glucopyranosiduronic acid (I) (10.6 mg, 0.0312 mmole) in 1 ml of tetrahydrofuran in a 5 ml reacti vial. Nitrogen gas was let into the vial and the latter was placed in an ice-bath. After 15 min isobutylchloroformiate (4.1 μl, 0.0312 mmole) and triethylamine (4.3 μl, 0.0312) were added and the reaction the acid (I) and isobutyl chloroformiate was completed in 30 min at 0° C. The reaction between KLH and the anhydride was run at 20 min a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com