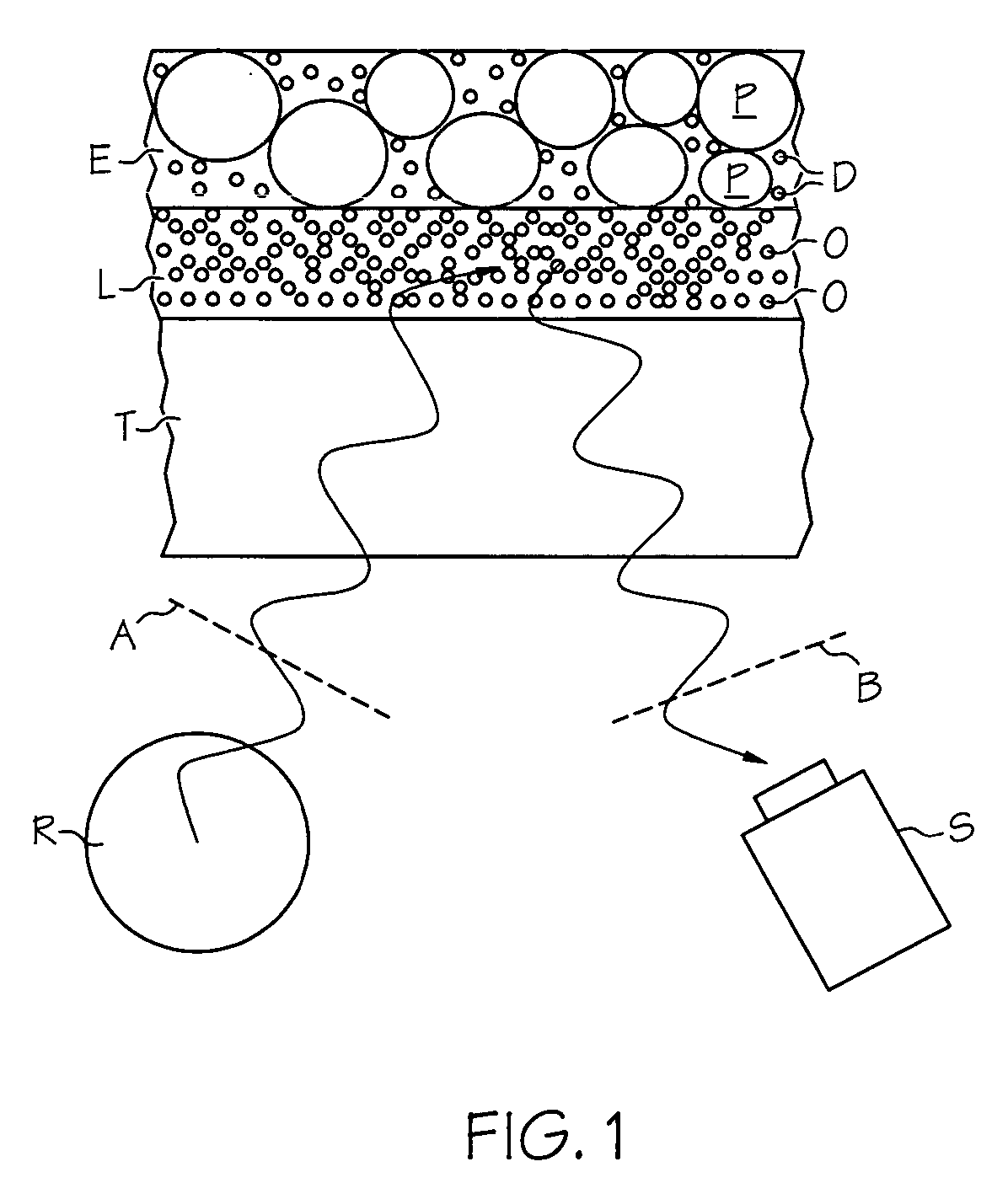
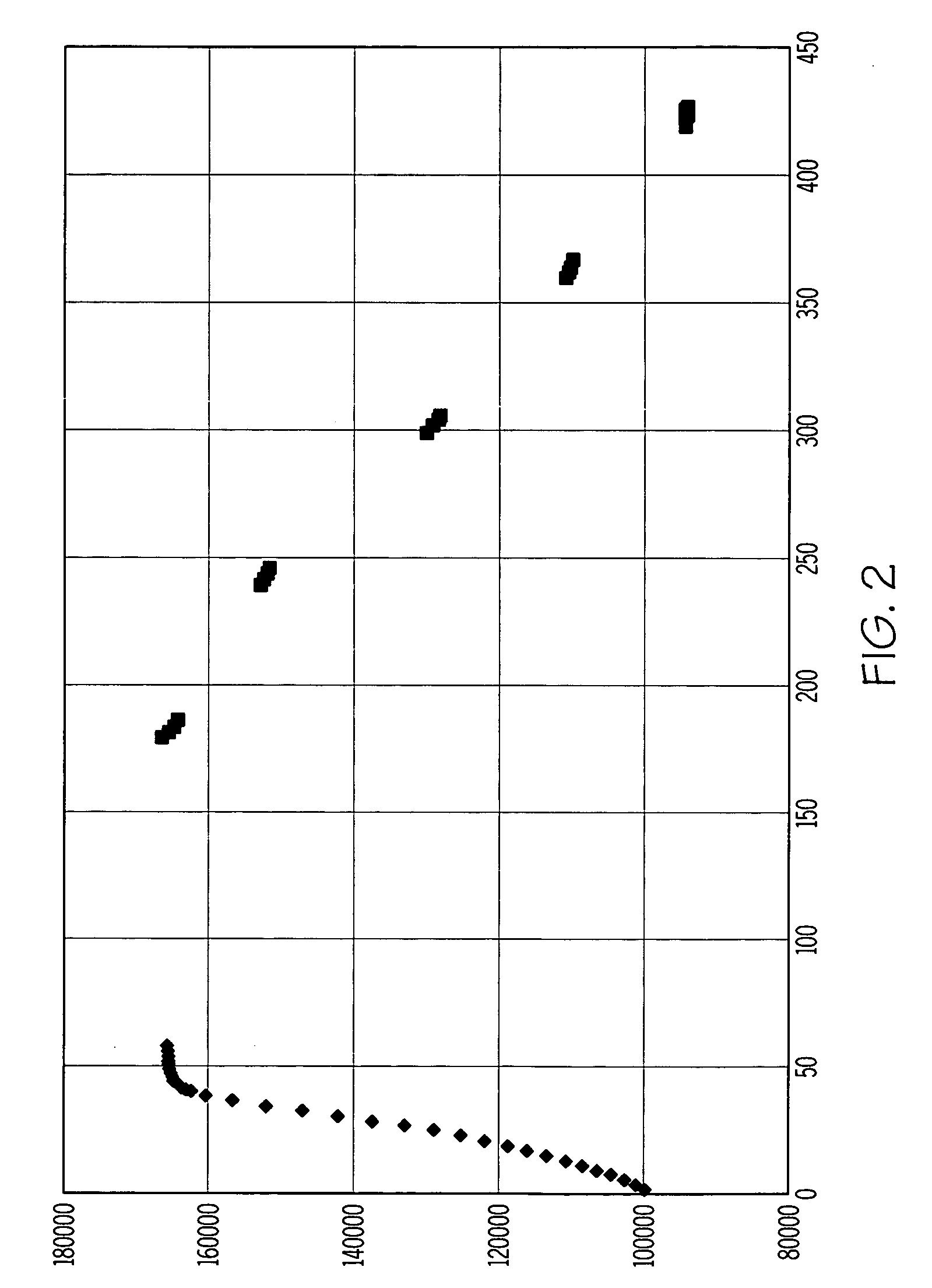
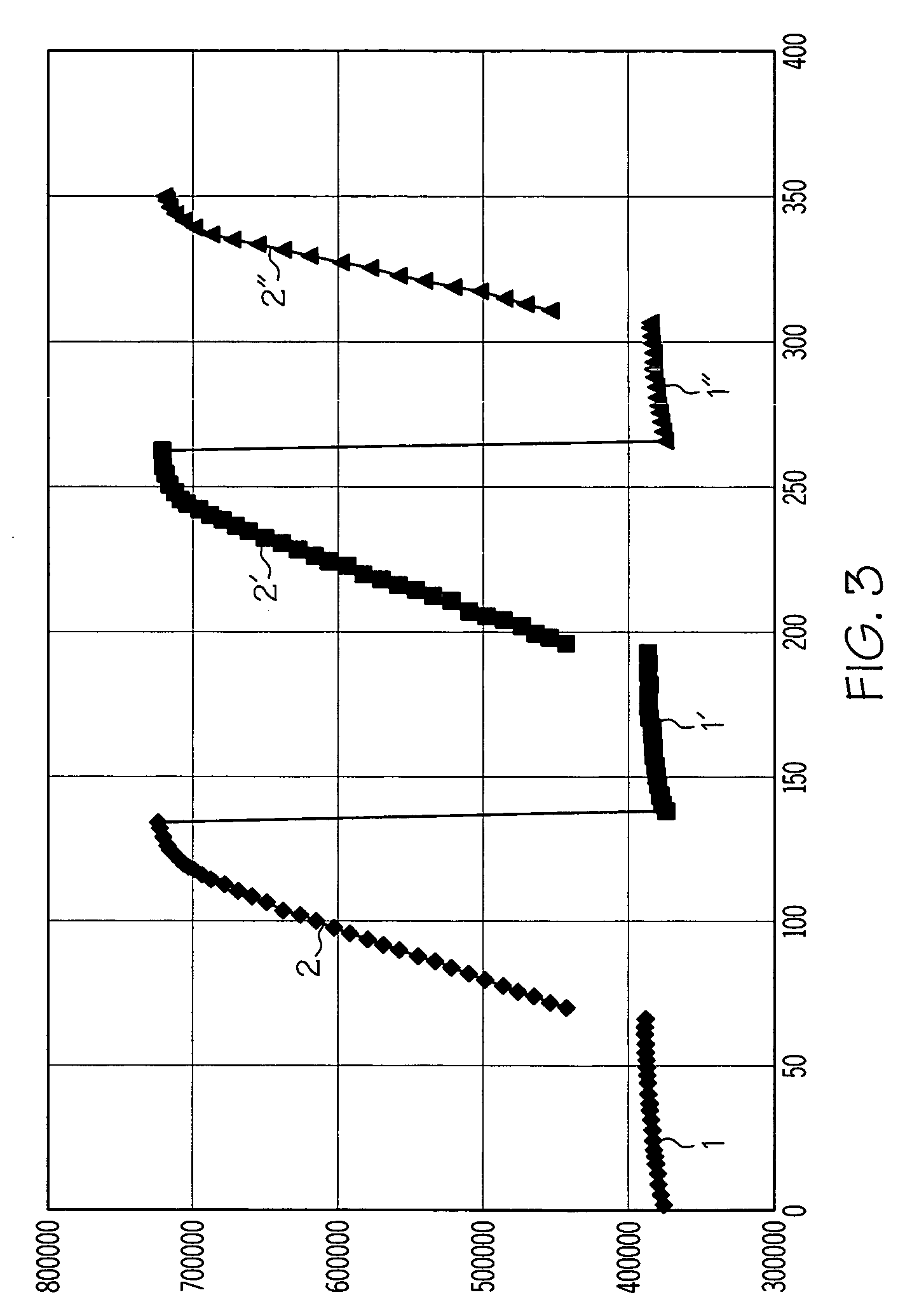
Diffusion layer for an enzyme-based sensor application
a sensor and enzyme technology, applied in biochemical apparatus and processes, specific use bioreactors/fermenters, after-treatment of biomass, etc., can solve the problems of inability to determine fast rates, too fast (a few seconds or less), depletion of oxygen in adjacent dye layers, etc., to achieve rapid oxygen recovery time, short wash time, and rapid hydration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Oxygen Dye Particles
[0074]
MaterialConcentrationTris(1,10-phenanthrpline)ruthenium(II)61.5gramschloride hydrate (cat. 34,371-4)Aldrich Chemical Co., Inc., 1001 West SaintPaul Ave., Milwaukee, WI 53233100 mM Phosphate buffer pH 7.57.5gramsSilica Gel (cat. 4115-100)2.25gramsWhatman Inc., 9 Bridewell Place,Clifton, NJ 07014
[0075] The dye tris-(1,10-phenanthroline) Ru (II) chloride was adsorbed onto silicagel particles according to a procedure published in: O. S. Wolfbeis, M. J. P. Leiner and H. E. Posch, “A new sensing material for optical oxygen measurement with the indicator embedded in an aqueous phase”, Microchim. Acta, III (1986) 359.
example 2
Preparation of the Oxygen Layer Mixture
[0076]
MaterialConcentrationO2 Ruthenium-silica dye particles0.5gramsPressure Sensitive Adhesive (cat. PSA590)4gramsGE Silicones, 260 Hudson River Road,Waterford, NY 12188Toluene2gramsAldrich Chemical Co., Inc., 1001 West SaintPaul Ave., Milwaukee, WI 53233
[0077] Add the Toluene to the Pressure Sensitive Adhesive and mix until homogeneous. Add this solution to the O2 indicator dye and mix for 16 hours.
example 3
Preparation of Enzyme-Carrying Hydrophilic Particles
[0078]
TABLE 1Glucose Oxidase ImmobilizationMaterialConcentrationCarboLink Coupling Gel (cat. 20391ZZ)5gramsPierce, 3747 North Meridian Road,Rockford, IL 61105Glucose Oxidase (cat. 1939998)0.15gramsRoche Molecular Biochemicals, 9115 HagueRoad, Indianapolis, IN 46250Sodium Periodate0.015gramsAldrich Chemical Co., Inc., 1001 West SaintPaul Ave., Milwaukee, WI 53233100 mM Phosphate buffer pH 7.515mLD-Salt Polyacrylamide Plastic Desalting10mL columncolumn (cat. 43243ZZ)Pierce, 3747 North Meridian Road,Rockford, IL 61105
[0079] The Sodium Periodate was added to 5 mL of 100 mM phosphate buffer and stirred for 10 minutes. To this solution was added the glucose oxidase, this solution was stirred at room temperature for 30 minutes. The solution was pippetted and added to the pre-filled polyacrylamide desalting column. The desalted glucose oxidase was collected in an appropriate container. The column was washed with 10 mL of 100 mM phosphate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com